Keywords
10]dodec-11-ene-35-diones
3
4-oxatetracyclo[5.3.2.02
5-cycloheptatrienes
6.08
7-substituted 1
biologically active compounds.
cycloaddition
maleic anhydride
Abstract
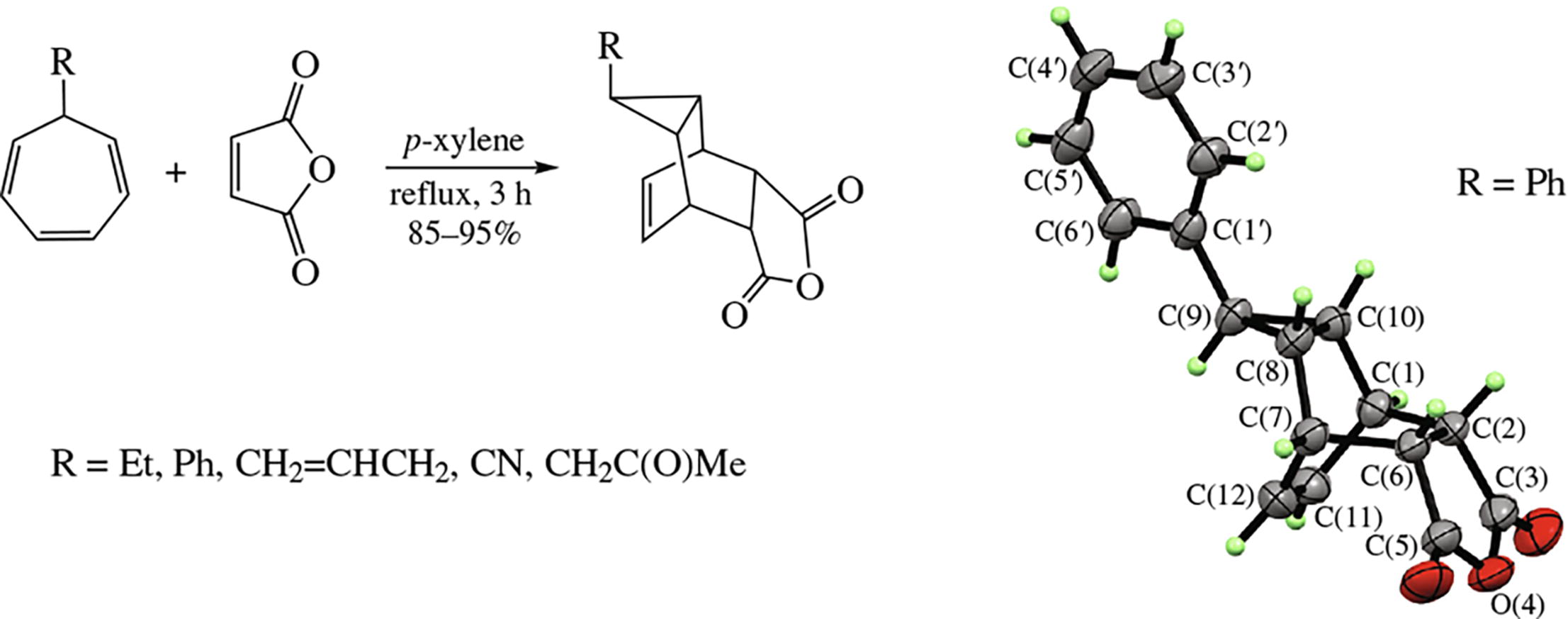
The [4+2]-cycloaddition of maleic anhydride to 7-substituted 1,3,5-cycloheptatrienes involves their 7-R-bicyclo[4.1.0]-hepta-2,4-diene tautomers and affords new 4-oxatetra- cyclo[5.3.2.02,6.08,10]dodec-11-ene-3,5-diones in 85-95% yields. The structure of the resulting adducts is proved using advanced 1D and 2D NMR techniques and X-ray diffraction analysis. The cycloaddition proceeds stereo specifically providing meso-(1S*,2S*,6R*,7R*,8R*,9-anti,10S*)-configuration in the products
References
.
Shybanov D.E., Kukushkin M.E., Tafeenko V.A., Zyk N.V., Grishin Y.K., Roznyatovsky V.A., Beloglazkina E.K.
Mendeleev Communications,
2021
.

Dyakonov V.A., Kadikova G.N., Dzhemilev U.M.
Russian Chemical Reviews,
2018
.

Frühauf H.
Chemical Reviews,
1997
.
![First Cobalt(I)-Catalyzed [6 + 2] Cycloadditions of Cycloheptatriene with Alkynes](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Achard M., Tenaglia A., Buono G.
Organic Letters,
2005
.

Petasis N.A., Patane M.A.
Tetrahedron,
1992
.

Lautens M., Klute W., Tam W.
Chemical Reviews,
1996
.

Rigby J.H.
Accounts of Chemical Research,
1993
.

Rigby J.H., Niyaz N.M., Short K., Heeg M.J.
Journal of Organic Chemistry,
1995
.
![Preparation of a Resin-Based Chromium Catalyst for Effecting [6π + 2π] Cycloaddition Reactions](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Rigby J.H., Kondratenko M.A., Fiedler C.
Organic Letters,
2000
.

Jordan R., Leeds J.M., Tyavanagimatt S., Hruby D.E.
Viruses,
2010
.
![N-(3,3a,4,4a,5,5a,6,6a-Octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol-2-(1H)-yl)carboxamides: Identification of Novel Orthopoxvirus Egress Inhibitors](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Bailey T.R., Rippin S.R., Opsitnick E., Burns C.J., Pevear D.C., Collett M.S., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Kern E.R., Keith K.A., Dai D., Yang G., Hruby D., et. al.
Journal of Medicinal Chemistry,
2007
.
![Chromium(0)-Promoted [6π + 2π] Cycloadditions of Allenes with Cycloheptatriene](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Rigby J.H., Laurent S.B., Kamal Z., Heeg M.J.
Organic Letters,
2008
.
![Highly Selective Cobalt-Mediated [6 + 2] Cycloaddition of Cycloheptatriene and Allenes](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Clavier H., Jeune K.L., Riggi I.D., Tenaglia A., Buono G.
Organic Letters,
2010
.
![Rhodium-Catalyzed [6 + 2] Cycloaddition of Internal Alkynes with Cycloheptatriene: Catalytic Study and DFT Calculations of the Reaction Mechanism](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Zhang X., Wang J., Zhao H., Zhao H., Wang J.
Organometallics,
2013
.
![The Synthesis of Bicyclo[4.2.1]nona-2,4,7-trienes by [6π + 2π]-Cycloaddition of 1-Substituted 1,3,5-Cycloheptatrienes Catalyzed by Titanium and Cobalt Complexes](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
D’yakonov V.A., Kadikova G.N., Nasretdinov R.N., Dzhemileva L.U., Dzhemilev U.M.
Journal of Organic Chemistry,
2019
.

Ito T., Harada S., Homma H., Takenaka H., Hirose S., Nemoto T.
Journal of the American Chemical Society,
2020
.
![Synthesis of Functionally Substituted Bicyclo[4.2.1]nona-2,4-dienes and Bicyclo[4.2.1]nona-2,4,7-trienes by Cobalt(I)-catalyzed [6π + 2π] Cycloaddition of 2-Tropylcyclohexanone](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Kadikova G.N., Dzhemileva L.U., D’yakonov V.A., Dzhemilev U.M.
ACS Omega,
2020
.

Kohler E.P., Tishler M., Potter H., Thompson H.T.
Journal of the American Chemical Society,
1939
.
![Synthetic studies on the ingenane diterpenes. Inter- and intramolecular [6 + 4] tropone-diene cycloaddition reactions](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Rigby J.H., Moore T.L., Rege S.
Journal of Organic Chemistry,
1986
.
![Synthetic studies on transition-metal-mediated higher order cycloaddition reactions: highly stereoselective construction of substituted bicyclo[4.4.1]undecane systems](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Rigby J.H., Ateeq H.S.
Journal of the American Chemical Society,
1990
.

Rigby J.H., Cuisiat S.V.
Journal of Organic Chemistry,
1993
.

Ishitobi H., Tanida H., Tori K., Tsuji T.
Bulletin of the Chemical Society of Japan,
1971
.
![Tricyclo[3.2.2.02,4]non-8-en-6,7-dicarbonic acid derivatives efficiently inhibits the replication of different orthopoxvirus species](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Selivanov B.A., Belanov E.F., Bormotov N.I., Balakhnin S.M., Serova O.A., Svyatchenko V.A., Kiselev N.N., Kazachinskaya E.I., Loktev V.B., Tikhonov A.Y.
Doklady Biological Sciences,
2011
.
![Synthesis and Antiviral Activity of Polycyclic N-Amidoimides Based on 4-Oxatetracyclo-[5.3.2.02, 6.08, 10]Dodec-11-Ene-3,5-Dione](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Selivanov B.A., Bormotov N.I., Shishkina L.N., Belanov E.F., Serova O.A., Kabanov A.S., Mazurkov O.Y., Tikhonov A.Y.
Pharmaceutical Chemistry Journal,
2019
.

Rigby J.H., Ateeq H.S., Krueger A.C.
Tetrahedron Letters,
1992
.
![[6+2]Cycloadditions catalyzed by titanium complexes](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Mach K., Antropiusová H., Petrusová L., Hanuš V., Tureček F., Sedmera P.
Tetrahedron,
1984
.

Rigby J.H.
Tetrahedron,
1999
.

Yu Z., Wang Y., Wang Y.
Chemistry - An Asian Journal,
2010
.
![PtCl2-Catalyzed [6+2] Cycloaddition of Alkynes Tethered to Cycloheptatriene](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Tenaglia A., Gaillard S.
Angewandte Chemie,
2008
.

Alder K., Jacobs G.
Chemische Berichte,
1953
.
Reichardt C., Yun K., Massa W., Schmidt R.E.
1985
.
![Ti-catalyzed [6π+2π] cycloadditions of allenes with 1,3,5-cycloheptatriene](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
D’yakonov V.A., Kadikova G.N., Dzhemilev U.M.
Tetrahedron Letters,
2011
.

Dong M., Zhang J., Peng X., Lu H., Yun L., Jiang S., Dai Q.
European Journal of Medicinal Chemistry,
2010
.
![Cobalt-Catalysed [6+2] Cycloaddition of Internal Alkynes and Terminal Alkenes with Cycloheptatriene](/storage/images/resized/xqixcltwJYe6H8Uco2JbAFfIOzt7UNKH0OcPOPzO_small_thumb.webp)
Cobalt-Catalysed [6+2] Cycloaddition of Internal Alkynes and Terminal Alkenes with Cycloheptatriene
Hilt G., Paul A., Hengst C.
Synthesis,
2009
.

Rigby J.H., Mann L.W., Myers B.J.
Tetrahedron Letters,
2001
.
![Rearrangement pathways in the bicyclo[4.4.1]undecane ring system](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Rigby J.H., Niyaz N.M., Bazin B.
Tetrahedron,
2002
.
Krinochkin A.P., Starnovskaya E.S., Valieva M.I., Kopchuk D.S., Santra S., Slepukhin P.A., Zyryanov G.V., Majee A., Chupakhin O.N.
Mendeleev Communications,
2022
.
Merkulova E.A., Kolobov A.V., Lyssenko K.A., Nenajdenko V.G.
Mendeleev Communications,
2022
.
![Titanium-catalyzed [6π+2π]-cycloaddition of Si-containing alkynes to bis(1,3,5-cycloheptatriene-7-yl)alkanes](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
D'yakonov V.A., Kadikova G.N., Nasretdinov R.N., Kolokol'tsev D.I., Dzhemilev U.M.
Tetrahedron Letters,
2017
.