Keywords
calix[4]resorcinols
condensation
conformation
rccc diastereoisomer
rctt diastereoisomer.
Abstract
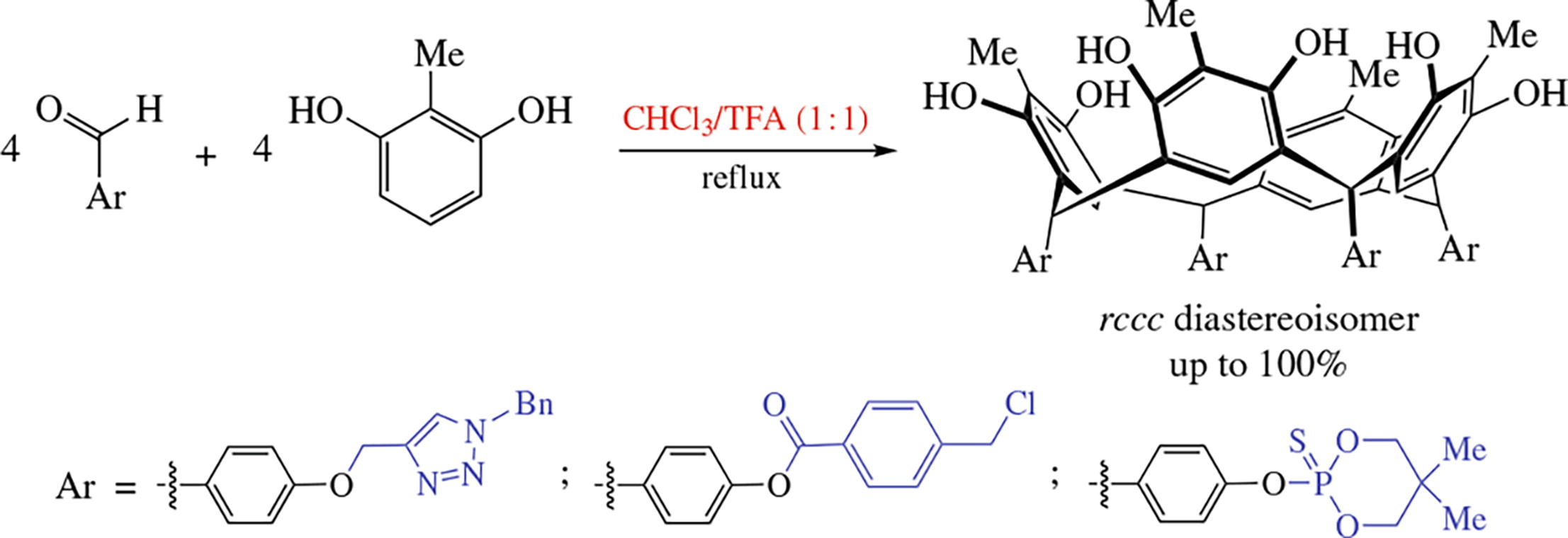
The use of excess of trifluoroacetic acid in chloroform (1 : 1, v/v) in a one-pot acid-catalyzed cyclocondensation of functionalized benzaldehydes with 2-methylresorcinol significantly improves the yield of calix[4]resorcinol rccc diastereoisomers compared to rctt ones. The fraction and yield of the rccc isomers sometimes approach 100% along with shortening the reaction time. The structure of new rccc diastereoisomer of chlorine-containing calix[4]resorcinol has been established by single crystal X-ray diffraction.
References
.

Farrugia L.J.
Journal of Applied Crystallography,
2012
.

Krause L., Herbst-Irmer R., Sheldrick G.M., Stalke D.
Journal of Applied Crystallography,
2015
.

Antipin I.S., Kazakova E.K., Habicher W.D., Konovalov A.I.
Russian Chemical Reviews,
1998
.
.

Knyazeva I.R., Burilov A.R., Pudovik M.A., Habicher W.D.
Russian Chemical Reviews,
2013
.
Knyazeva I.R., Syakaev V.V., Lodochnikova O.A., Burilov A.R.
Mendeleev Communications,
2019
.
![Chemistry of calix[4]resorcinarenes](/storage/images/resized/9Mus3KG1Tkd7Bwaurt8H3RwWh0CxRlGoO6ng9UK1_small_thumb.webp)
Jain V.K., Kanaiya P.H.
Russian Chemical Reviews,
2011
.

Tunstad L.M., Tucker J.A., Dalcanale E., Weiser J., Bryant J.A., Sherman J.C., Helgeson R.C., Knobler C.B., Cram D.J.
Journal of Organic Chemistry,
1989
.

Thoden van Velzen E.U., Engbersen J.F., Reinhoudt D.N.
Journal of the American Chemical Society,
1994
.

Hoegberg A.G.
Journal of Organic Chemistry,
1980
.
![Rapid synthesis of dendrimers based on calix[4]resorcinarenes](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Yamakawa Y., Ueda M., Nagahata R., Takeuchi K., Asai M.
Journal of the Chemical Society Perkin Transactions 1,
1998
.
![Synthesis of rctt, rccc, and rcct diastereomers of calix[4]methylresorcinarenes based on p-tolualdehyde. X-ray diffraction study of the rcct isomer. Formation of rctt and rccc cavitands in a cone conformation](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Prosvirkin A.V., Kazakova E.K., Gubaidullin A.T., Litvinov I.A., Gruner M., Habicher W.D., Konovalov A.I.
Russian Chemical Bulletin,
2005
.
![Synthesis of rccc- and rctt-diastereoisomers of novel triazole-containing calix[4]resorcinols](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Knyazeva I.R., Mukhamedyanova K.M., Syakaev V.V., Gubaidullin A.T., Habicher W.D., Burilov A.R.
Tetrahedron Letters,
2018
.

Timmerman P., Verboom W., Reinhoudt D.N.
Tetrahedron,
1996
.
Knyazeva I.R., Abdrafikova D.K., Mukhamedyanova K.M., Syakaev V.V., Gabidullin B.M., Gubaidullin A.T., Habicher W.D., Burilov A.R., Pudovik M.A.
Mendeleev Communications,
2017
.
![One-step synthesis of rccc- and rctt-diastereomers of novel calix[4]resorcinols based on a para-thiophosphorylated derivative of benzaldehyde](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Knyazeva I.R., Sokolova V.I., Gruner M., Habicher W.D., Syakaev V.V., Khrizanforova V.V., Gabidullin B.M., Gubaidullin A.T., Budnikova Y.H., Burilov A.R., Pudovik M.A.
Tetrahedron Letters,
2013
.
![Condensation of resorcinol with phosphorylated acetals, synthesis of calix[4]resorcinolarenes with phosphorus-containing alkyl fragments in the lower rim](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Burilov A.R., Volodina Y.M., Popova E.V., Gazizov A.S., Knyazeva I.R., Pudovik M.A., Habicher V.D., Konovalov A.I.
Russian Journal of General Chemistry,
2006
.
![Selective formation of the rctt chair stereoisomers of octa-O-alkyl resorcin[4]arenes using Brønsted acid catalysis](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Moore D., Watson G.W., Gunnlaugsson T., Matthews S.E.
New Journal of Chemistry,
2008
.
![Cyclooligomeric phenol-aldehyde condensation products. 2. Stereoselective synthesis and DNMR study of two 1,8,15,22-tetraphenyl[14]metacyclophan-3,5,10,12,17,19,24,26-octols](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Hoegberg A.G.
Journal of the American Chemical Society,
1980
.

Iwanek W.
Tetrahedron,
1998
.
![Molecular iodine: An efficient and environment-friendly catalyst for the synthesis of calix[4]resorcinarenes](/storage/images/resized/o8UHDgKqyqq8rLGkA2mcwjMSf5fWh8DCcBP1VPsn_small_thumb.webp)
Darvish F., Khazraee S.
Comptes Rendus Chimie,
2014
.

Gramage-Doria R., Armspach D., Matt D.
Coordination Chemistry Reviews,
2013
.
![Lanthanide(III) nitrobenzenesulfonates and p-toluenesulfonate complexes of lanthanide(III), iron(III), and copper(II) as novel catalysts for the formation of calix[4]resorcinarene](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Deleersnyder K., Mehdi H., Horváth I.T., Binnemans K., Parac-Vogt T.N.
Tetrahedron,
2007
.
Knyazeva I.R., Syakaev V.V., Habicher W.D., Burilov A.R.
Mendeleev Communications,
2022
.
![Applications of supramolecular capsules derived from resorcin[4]arenes, calix[n]arenes and metallo-ligands: from biology to catalysis](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Gangemi C.M., Pappalardo A., Trusso Sfrazzetto G.
RSC Advances,
2015
.
![Recent advances in upper rim functionalization of resorcin[4]arene derivatives: Synthesis and applications](/storage/images/resized/5YZtvLvkPZuc2JHOaZsjCvGSHFCuC3drUwN3YAc5_small_thumb.webp)
Gajjar J.A., Vekariya R.H., Parekh H.M.
Synthetic Communications,
2020
.
Knyazeva I.R., Syakaev V.V., Habicher W.D., Burilov A.R.
Mendeleev Communications,
2023