Keywords
carbon family
catenation
group 14 chemistry and reactivity
ionization energy
radical cations.
Abstract
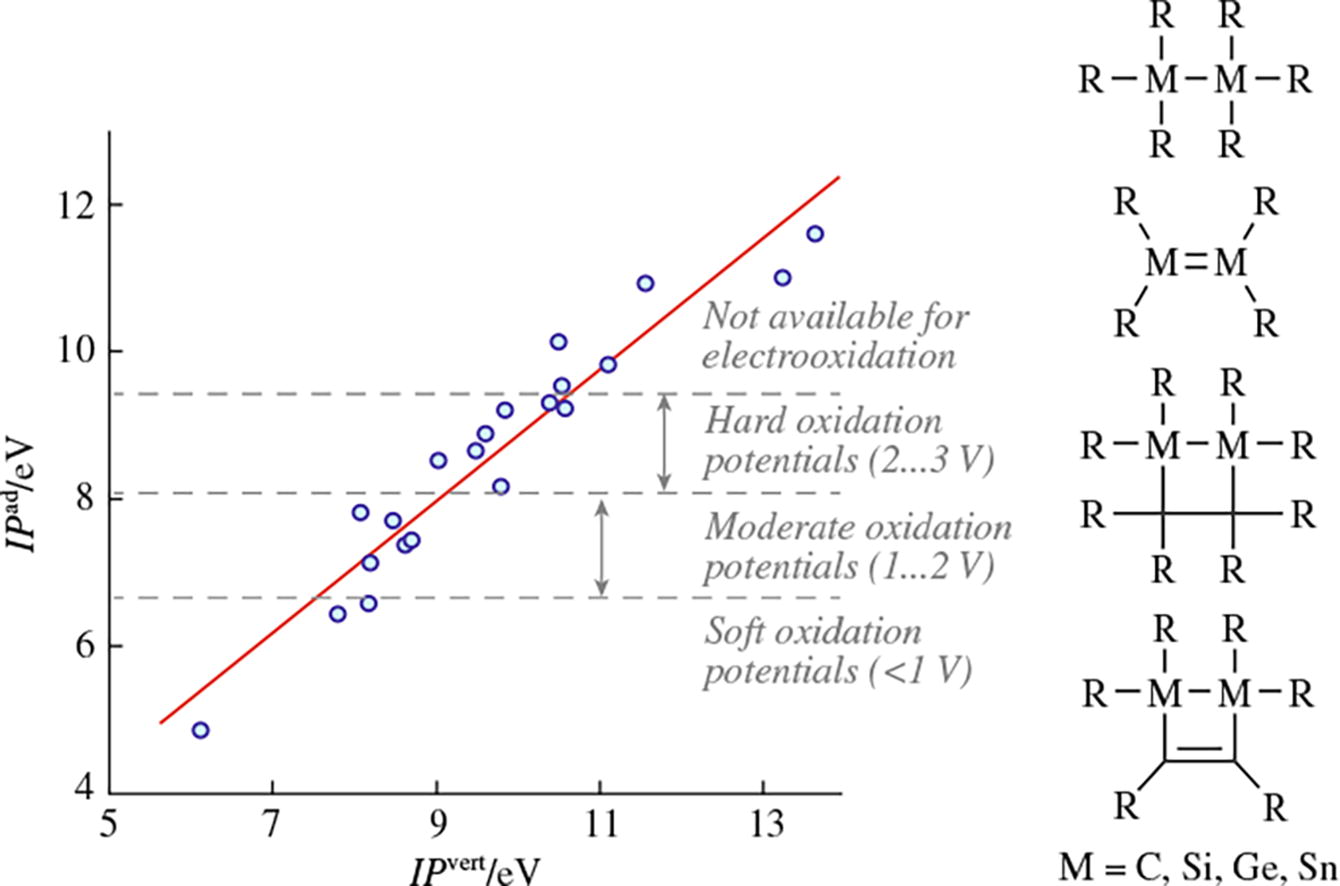
Adiabatic ionization energies for a series of acyclic and cyclic compounds containing M-M and M=M (M = C, Si, Ge, and Sn) bonds were calculated using DFT-TPSS/D3/def2-TZVPP and DFT-PBE0-DH/def2-TZVPP. Their changes when moving down group 14, as well as the influence of the substituent nature on them, were discussed.
References
.

Weigend F., Ahlrichs R.
Physical Chemistry Chemical Physics,
2005
.

Grimme S., Antony J., Ehrlich S., Krieg H.
Journal of Chemical Physics,
2010
.
![The Structure of Fractionally Charged Tetracyanobenzenen− Present in [TCNB]32−](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Bagnato J.D., Shum W.W., Strohmeier M., Grant D.M., Arif A.M., Miller J.S.
Angewandte Chemie - International Edition,
2006
.

Arsenyeva K.V., Pashanova K.I., Trofimova O.Y., Ershova I.V., Chegerev M.G., Starikova A.A., Cherkasov A.V., Syroeshkin M.A., Kozmenkova A.Y., Piskunov A.V.
New Journal of Chemistry,
2021
.

.

Klare H.F., Albers L., Süsse L., Keess S., Müller T., Oestreich M.
Chemical Reviews,
2021
.

Shangin P.G., Akyeva A.Y., Vakhrusheva D.M., Minyaev M.E., Mankaev B.N., Balycheva V.A., Lalov A.V., Egorov M.P., Karlov S.S., Syroeshkin M.A.
Organometallics,
2023
.

Mochida K., Hodota C., Hata R., Fukuzumi S.
Organometallics,
1993
.

Ando W., Kako M., Akasaka T.
Journal of the Chemical Society Chemical Communications,
1992
.

Fischer R.C., Power P.P.
Chemical Reviews,
2010
.

Marenich A.V., Cramer C.J., Truhlar D.G.
Journal of Physical Chemistry B,
2009
.

Sheremetev A.B., Lyalin B.V., Kozeev A.M., Palysaeva N.V., Struchkova M.I., Suponitsky K.Y.
RSC Advances,
2015
.

Fa W., Zeng X.C.
Chemical Communications,
2014
.

Power P.P.
Chemical Reviews,
1999
.

Luca O.R., Gustafson J.L., Maddox S.M., Fenwick A.Q., Smith D.C.
Organic Chemistry Frontiers,
2015
.

Neese F., Wennmohs F., Becker U., Riplinger C.
Journal of Chemical Physics,
2020
.

Holmes J.L., Lossing F.P.
Organic Mass Spectrometry,
1991
.

Zhan C., Nichols J.A., Dixon D.A.
Journal of Physical Chemistry A,
2003
.

Wendel D., Szilvási T., Jandl C., Inoue S., Rieger B.
Journal of the American Chemical Society,
2017
.

Akyeva A.Y., Kansuzyan A.V., Vukich K.S., Kuhn L., Saverina E.A., Minyaev M.E., Pechennikov V.M., Egorov M.P., Alabugin I.V., Vorobyev S.V., Syroeshkin M.A.
Journal of Organic Chemistry,
2022
.

Kozmenkova A.Y., Timofeeva V.A., Mankaev B.N., Lalov A.V., Saverina E.A., Egorov M.P., Karlov S.S., Syroeshkin M.A.
European Journal of Inorganic Chemistry,
2021
.

Fedyushin P.A., Zayakin I.A., Tolstikov S.E., Lalov A.V., Akyeva A.Y., Syroeshkin M.A., Romanenko G.V., Tretyakov E.V., Egorov M.P., Ovcharenko V.I.
Russian Chemical Bulletin,
2022
.

Bouhadir G., Bourissou D.
Chemical Society Reviews,
2004
.

Zeitouny J., Jouikov V.
Physical Chemistry Chemical Physics,
2009
.

Wedler H.B., Wendelboe P., Tantillo D.J., Power P.P.
Dalton Transactions,
2020
.

Saalfeld F.E., Svec H.J.
Inorganic Chemistry,
1963
.

Robin M.B., Taylor G.N., Kuebler N.A., Bach R.D.
Journal of Organic Chemistry,
1973
.

Gaidis J.M., Briggs P.R., Shannon T.W.
The Journal of Physical Chemistry,
1971
.

Wiberg K.B., Ellison G.B., Wendoloski J.J., Brundle C.R., Kuebler N.A.
Journal of the American Chemical Society,
1976
.

Mollere P.D., Houk K.N., Bomse D.S., Morton T.H.
Journal of the American Chemical Society,
1976
.

Bock H., Ensslin W., Feher F., Freund R.
Journal of the American Chemical Society,
1976
.

Perdew J.P., Ruzsinszky A., Csonka G.I., Constantin L.A., Sun J.
Physical Review Letters,
2009
.
![Erratum: Workhorse Semilocal Density Functional for Condensed Matter Physics and Quantum Chemistry [Phys. Rev. Lett.103, 026403 (2009)]](/storage/images/resized/nrK64iXHTzj43wMrfN1ZoUQ0vanswGzWPN45K3jA_small_thumb.webp)
Perdew J.P., Ruzsinszky A., Csonka G.I., Constantin L.A., Sun J.
Physical Review Letters,
2011
.

Mochida K., Worley S.D., Kochi J.K.
Bulletin of the Chemical Society of Japan,
1985
.

Cradock S., Ebsworth E.A., Whiteford R.A.
Journal of the Chemical Society Dalton Transactions,
1973
.

Bieri G., Burger F., Heilbronner E., Maier J.P.
Helvetica Chimica Acta,
1977
.

Potzinger P., Ritter A., Krause J.
Zeitschrift fur Naturforschung - Section A Journal of Physical Sciences,
1975
.

Szepes L., Korányi T., Náray-Szabó G., Modelli A., Distefano G.
Journal of Organometallic Chemistry,
1981
.

Ruscic B., Berkowitz J.
Journal of Chemical Physics,
1991
.

De Proft F., Geerlings P.
Journal of Chemical Physics,
1997
.

Garcia E., Bard A.J.
Journal of the Electrochemical Society,
1990
.
![Reactivity of the Isolable Disilene R*PhSidSiPhR* (R*=SitBu3) Compounds of Silicon, Part 155. Unsaturated silicon compounds, Part 62. For parts 154 and 61 see ref. [2].](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Wiberg N., Niedermayer W., Polborn K., Mayer P.
Chemistry - A European Journal,
2002
.

Bande A., Michl J.
Chemistry - A European Journal,
2009
.

Heublein G., Spange S.
Journal für praktische Chemie,
1980
.

Inghram M.G., Hanson G.R., Stockbauer R.
International Journal of Mass Spectrometry and Ion Physics,
1980
.

Branton G.R., Frost D.C., Makita T., McDowell C.A., Stenhouse I.A.
Journal of Chemical Physics,
1970
.

Egorov M.P., Jouikov V.V., Nikolaevskaya E.N., Syroeshkin M.A.
2023