Keywords
crystal structure.
nucleophilic substitution
perfluroarenes
synthesis
thioacetic acid
thiols
Abstract
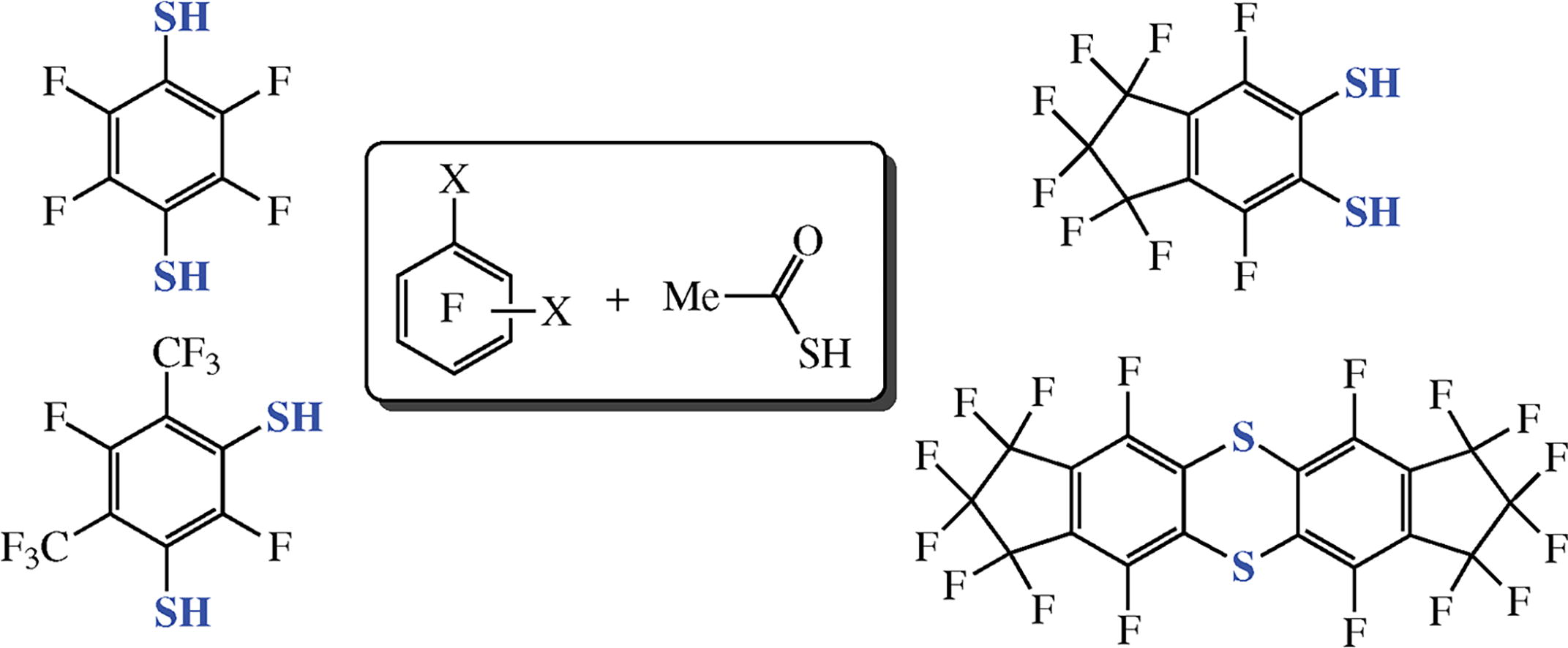
The direction of the reaction between substituted perfluorobenzenes and thioacetic acid depends on the location of the substituents in perfluoroaromatic ring. Hexafluorobenzene reacts at positions 1 and 4, perfluoro-m-xylene reacts at positions 4 and 6, while perfluoroindane reacts at positions 5 and 6 to give the corresponding dithiols with para-, meta- and ortho-location of the thiol groups, respectively. Domino-reaction of perfluoroindane-5-thiol and its acetyl thioester affords hexadecafluoro-2,12-dithia-pentacyclo[11.7.0.03,11.05,9.015,19]icosa-1(13),3,5(9),10,14,19-hexaene
References
.

Chivers T., Parvez M., Vargas-Baca I., Schatte G.
Canadian Journal of Chemistry,
1998
.

Umemoto T., Garrick L.M., Saito N.
Beilstein Journal of Organic Chemistry,
2012
.

Kovtonyuk V.N., Gatilov Y.V., Nikul’shin P.V., Bredikhin R.A.
Molecules,
2021
.

Skowron P., Dumartin M., Jeamet E., Perret F., Gourlaouen C., Baudouin A., Fenet B., Naubron J., Fotiadu F., Vial L., Leclaire J.
Journal of Organic Chemistry,
2016
.

Eady S.C., MacInnes M.M., Lehnert N.
Inorganic Chemistry,
2017
.

Zaffaroni R., Dzik W.I., Detz R.J., van der Vlugt J.I., Reek J.N.
European Journal of Inorganic Chemistry,
2019
.

Zhang G., Chan J.M.
Journal of Materials Chemistry C,
2017
.

Raasch M.S.
Journal of Organic Chemistry,
1979
.

Koh M.J., Khan R.K., Torker S., Yu M., Mikus M.S., Hoveyda A.H.
Nature,
2015