Keywords
azacrown ether
cyclopenteno-13-crown-3 ether.
hydrazone
Nazarov reaction
piperidone
Abstract
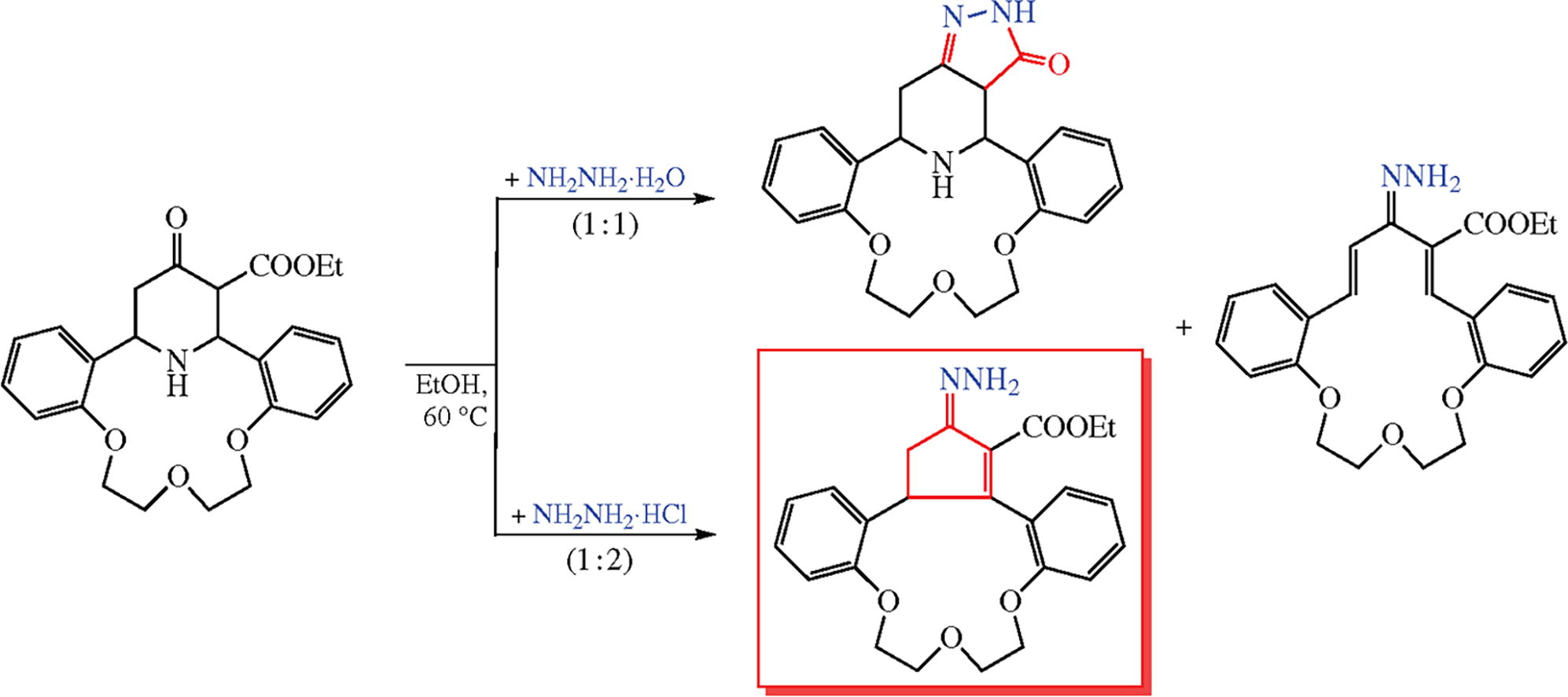
Reactions of aza-14-crown-4 incorporating 4-oxopiperidine-3-carboxylate moiety with hydrazine and hydroxylamine proceed at the functional groups. One unexpected product, cyclopenteno-13-crown-3 ether derivative, is formed when hydrazine hydrochloride is used, its formation occurring via deamination of the piperidine followed by the Nazarov reaction of the intermediate hydrazono diene. According to the QSAR through ADMET calculation, the synthesized compounds may show high potential for biological studies.
References
.

Daina A., Michielin O., Zoete V.
Scientific Reports,
2017
.

Levov A.N., Le An’ T., Komarova A.I., Strokina V.M., Soldatenkov A.T., Khrustalev V.N.
Russian Journal of Organic Chemistry,
2008
.

Frontier A.J., Collison C.
Tetrahedron,
2005
.

He W., Sun X., Frontier A.J.
Journal of the American Chemical Society,
2003
.
Vystorop I.V., Konovalova N.P., Sashenkova T.E., Berseneva E.N., Chernyak A.V., Fedorov B.S., Kostyanovsky R.G.
Mendeleev Communications,
2011
.

Liang G., Gradl S.N., Trauner D.
Organic Letters,
2003
.

Levov A.N., Le Tuan A., Soldatenkov A.T., Khieu C.K., Khrustalev V.N.
Russian Journal of Organic Chemistry,
2008
.

Easwaramoorthi S., Umamahesh B., Cheranmadevi P., Rathore R.S., Iyer Sathiyanarayanan K.
RSC Advances,
2013
.

Baliah V., Jeyaraman R., Chandrasekaran L.
Chemical Reviews,
1983
.

Anusevičius K., Mickevičius V., Belyakov S., Šiugždaitė J., Kantminienė K.
Monatshefte fur Chemie,
2014
.
Le A.T., Dao N.T., Truong H.H., Do T.T., Polyakova E.I., Lin’ko I.V., Dorovatovskii P.V., Khrustalev V.N.
Mendeleev Communications,
2019
.
Le A.T., Tran V.T., Truong H.H., Nguyen L.M., Luong D.M., Do T.T., Nguyen D.T., Dao N.T., Le D.T., Soldatenkov A.T., Khrustalev V.N.
Mendeleev Communications,
2019
.
Le T.A., Truong H.H., Thi T.P., Thi N.D., To H.T., Thi H.P., Soldatenkov A.T.
Mendeleev Communications,
2015
.
Levov A.N., Strokina V.M., Anh L.T., Komarova A.I., Soldatenkov A.T., Khrustalev V.N.
Mendeleev Communications,
2006
.

.

Prostakov N.S., Gaivoronskaya L.A.
Russian Chemical Reviews,
1978
.

Dao N.T., Khrustalev V.N., Polyakova E.I., Le A.T.
ChemistrySelect,
2022
.

Zhu W., Mena M., Jnoff E., Sun N., Pasau P., Ghosez L.
Angewandte Chemie - International Edition,
2009
.
Makarov M.V., Skvortsov E.A., Brel V.K.
Mendeleev Communications,
2015
.

Gopalakrishnan M., Kanagarajan V., Thanusu J.
Green Chemistry Letters and Reviews,
2008
.
![Facile Synthesis of 2-Amino-5,6,7,8-tetrahydro-5,7-diarylpyrido[4,3-d]pyrimidin-4-ols](/storage/images/resized/5YZtvLvkPZuc2JHOaZsjCvGSHFCuC3drUwN3YAc5_small_thumb.webp)
Amutha P., Nagarajan S.
Synthetic Communications,
2009
.

Umamatheswari S., Balaji B., Ramanathan M., Kabilan S.
Bioorganic and Medicinal Chemistry Letters,
2010
.

Rameshkumar N., Veena A., Ilavarasan R., Adiraj M., Shanmugapandiyan P., Sridhar S.K.
Biological and Pharmaceutical Bulletin,
2003