Keywords
benzothiadiazole
cross-coupling
luminescence
luminophore
OLED.
Abstract
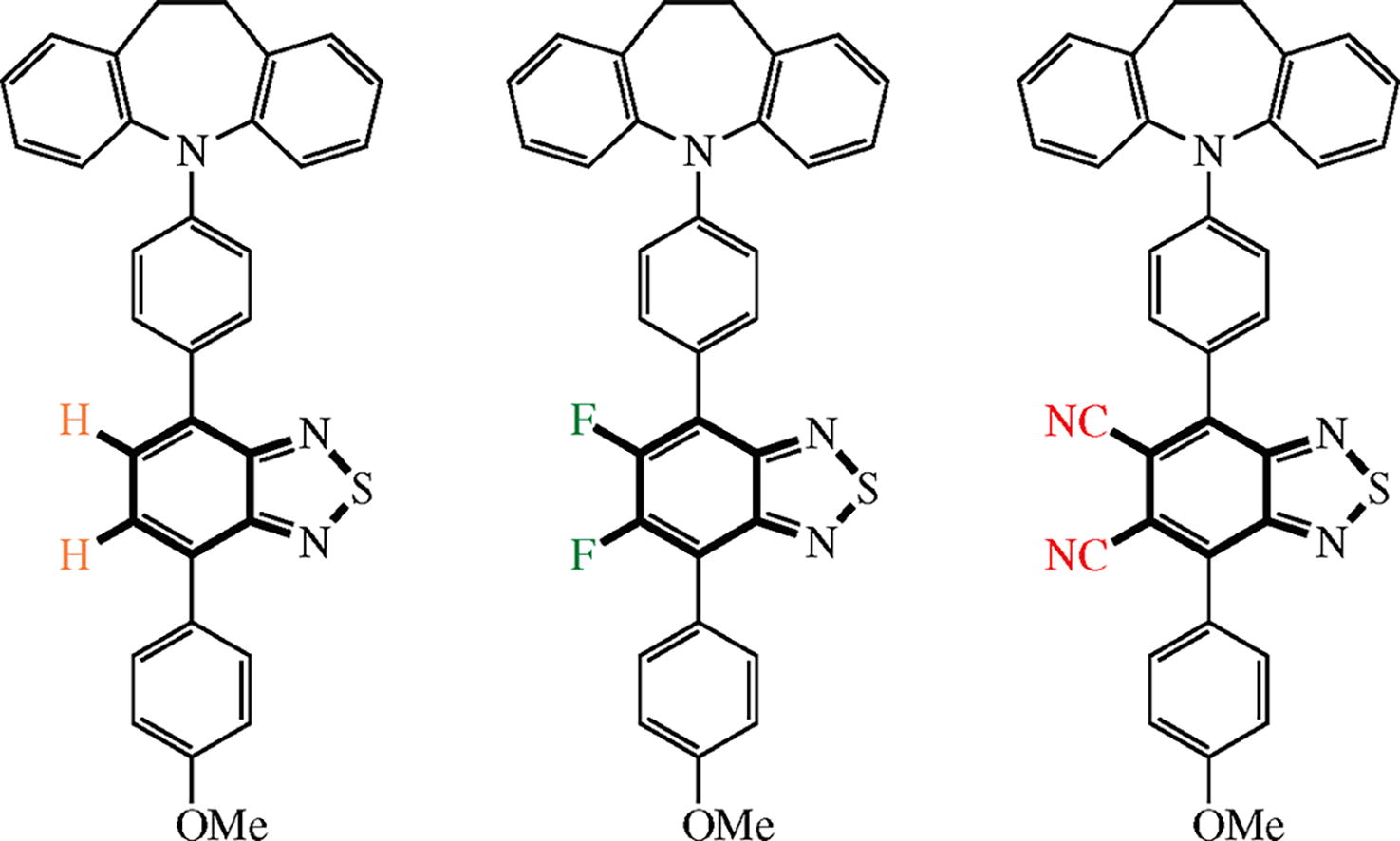
Three new 2,1,3-benzothiadiazole-containing luminophores with different electron deficiency of acceptor block have been prepared via the Pd-catalyzed Suzuki cross-coupling and nucleophilic fluorine substitution with cyano group. Photophysical and electroluminescent properties of the compounds obtained were investigated to estimate their potential for optoelectronic applications. The introduction of two fluorine atoms in the benzothiadiazole moiety leads to the hypsochromic shift of the electroluminescent emission, whereas the incorporation of two cyano groups shifts it into the long-wave region. All synthesized compounds were used as emissive layers in OLED devises.
References
.

Zhu W., Wu Y., Wang S., Li W., Li X., Chen J., Wang Z., Tian H.
Advanced Functional Materials,
2010
.

Neto B.A., Carvalho P.H., Correa J.R.
Accounts of Chemical Research,
2015
.

Kato S., Matsumoto T., Shigeiwa M., Gorohmaru H., Maeda S., Ishi-i T., Mataka S.
Chemistry - A European Journal,
2006
.

Zhang M., Tsao H.N., Pisula W., Yang C., Mishra A.K., Müllen K.
Journal of the American Chemical Society,
2007
.

Li Y.
Accounts of Chemical Research,
2012
.

Wang T., Yang C., Chuang Y.
RSC Advances,
2016
.

Çakal D., Ercan Y.E., Önal A.M., Cihaner A.
Dyes and Pigments,
2020
.

Sukhikh T.S., Ogienko D.S., Bashirov D.A., Konchenkoa S.N.
Russian Chemical Bulletin,
2019
.

Guo J., Wang S., Dai N., Teo Y.N., Kool E.T.
Proceedings of the National Academy of Sciences of the United States of America,
2011
.

Neo W.T., Ong K.H., Lin T.T., Chua S., Xu J.
Journal of Materials Chemistry C,
2015
.

Sonar P., Singh S.P., Li Y., Soh M.S., Dodabalapur A.
Advanced Materials,
2010
.

Nielsen C.B., White A.J., McCulloch I.
Journal of Organic Chemistry,
2015
.
Gribanov P.S., Lypenko D.A., Dmitriev A.V., Pozin S.I., Topchiy M.A., Asachenko A.F., Loginov D.A., Osipov S.N.
Mendeleev Communications,
2021
.

Gribanov P.S., Loginov D.A., Lypenko D.A., Dmitriev A.V., Pozin S.I., Aleksandrov A.E., Tameev A.R., Martynov I.L., Chernyadyev A.Y., Osipov S.N.
Molecules,
2021
.

Tsao H.N., Cho D.M., Park I., Hansen M.R., Mavrinskiy A., Yoon D.Y., Graf R., Pisula W., Spiess H.W., Müllen K.
Journal of the American Chemical Society,
2011
.

Carsten B., Szarko J.M., Son H.J., Wang W., Lu L., He F., Rolczynski B.S., Lou S.J., Chen L.X., Yu L.
Journal of the American Chemical Society,
2011
.

Qiao Y., Guo Y., Yu C., Zhang F., Xu W., Liu Y., Zhu D.
Journal of the American Chemical Society,
2012
.
![Critical Role of Molecular Symmetry for Charge Transport Properties: A Paradigm Learned from Quinoidal Bithieno[3,4-b]thiophenes](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Ren L., Yuan D., Gann E., Guo Y., Thomsen L., McNeill C.R., Di C., Yi Y., Zhu X., Zhu D.
Chemistry of Materials,
2017
.
![Ultrafast Intramolecular and Solvation Dynamics in 4,7-Bis (4,5-dibutylbenzo[1,2-b:4,3-b′]bisthiophene[1,2-b:4,3-b′]bisthiophen-2-yl)-2,1,3-benzothiadiazole](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Patrizi B., Iagatti A., Abbondanza L., Bussotti L., Zanardi S., Salvalaggio M., Fusco R., Foggi P.
Journal of Physical Chemistry C,
2019
.

Wudarczyk J., Papamokos G., Margaritis V., Schollmeyer D., Hinkel F., Baumgarten M., Floudas G., Müllen K.
Angewandte Chemie - International Edition,
2016
.
![A Simple and Strong Electron‐Deficient 5,6‐Dicyano[2,1,3]benzothiadiazole‐Cored Donor‐Acceptor‐Donor Compound for Efficient Near Infrared Thermally Activated Delayed Fluorescence](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Kumsampao J., Chaiwai C., Chasing P., Chawanpunyawat T., Namuangruk S., Sudyoadsuk T., Promarak V.
Chemistry - An Asian Journal,
2020
.

Ding B., Kim G., Kim Y., Eisner F.D., Gutiérrez‐Fernández E., Martín J., Yoon M., Heeney M.
Angewandte Chemie - International Edition,
2021
.

Babudri F., Farinola G.M., Naso F., Ragni R.
Chemical Communications,
2007
.

Miao Y., Du X., Wang H., Liu H., Jia H., Xu B., Hao Y., Liu X., Li W., Huang W.
RSC Advances,
2015
.

Du J., Biewer M.C., Stefan M.C.
Journal of Materials Chemistry A,
2016
.

Müller C.D., Falcou A., Reckefuss N., Rojahn M., Wiederhirn V., Rudati P., Frohne H., Nuyken O., Becker H., Meerholz K.
Nature,
2003
.

Gribanov P.S., Vorobyeva D.V., Tokarev S.D., Loginov D.A., Danshina A.A., Masoud S.M., Osipov S.N.
Asian Journal of Organic Chemistry,
2022
.

Kim B.S., Lee J.Y.
Advanced Functional Materials,
2014
.

Bezvikonnyi O., Gudeika D., Volyniuk D., Rutkis M., Grazulevicius J.V.
Dyes and Pigments,
2020
.

Tomkeviciene A., Bartiuk T., Bucinskas A., Grazulevicius J.V., Jankauskas V.
Reactive and Functional Polymers,
2011