Keywords
benzimidazole derivatives
nitroanilines
recyclization–isomerization
regioselective reduction
titanium trichloride.
Abstract
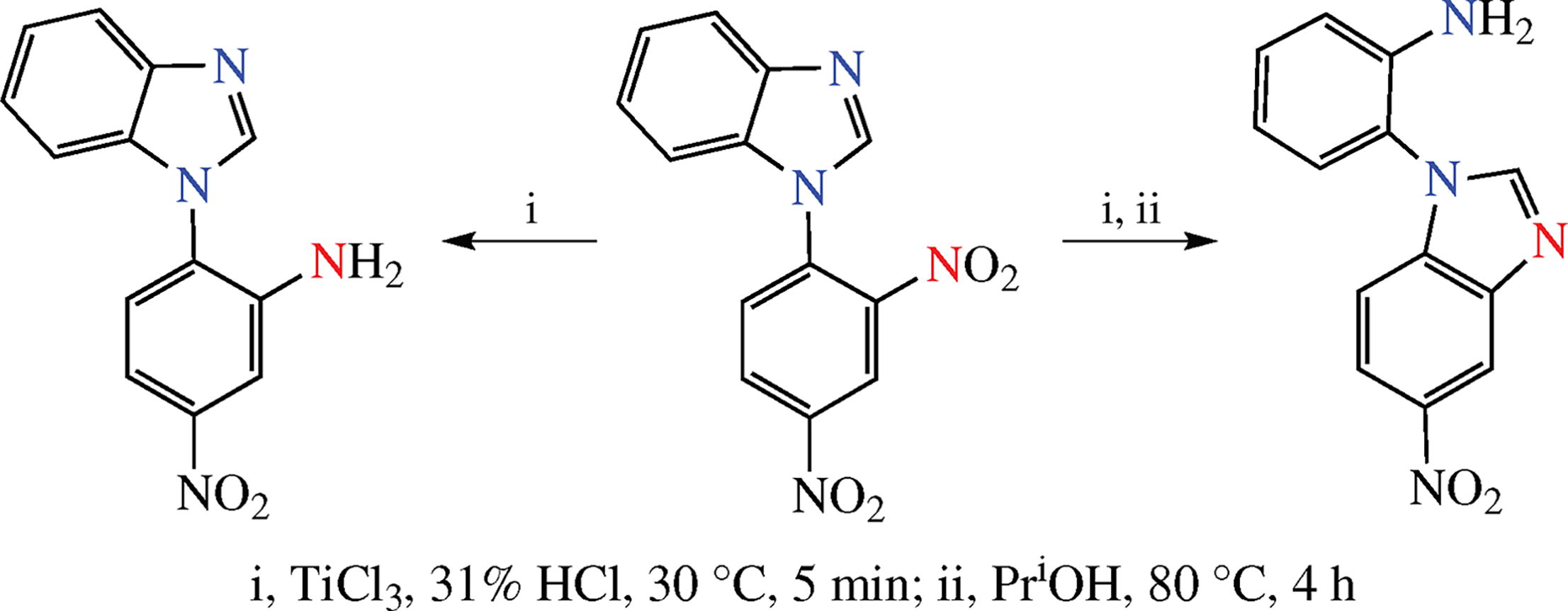
The efficiency of two strategies for the synthesis of N-(2-aminophenyl)-5-nitrobenzimidazole derivatives was studied. The promising route involves the stages of 1-(2,4-dinitro-phenyl)-1H-benzimidazole monoreduction at 2-positioned nitro group and tandem conversion involving recyclization of the intermediate N-(2-amino-4-nitrophenyl)-5-nitrobenz-imidazole.
References
.
![Synthesis and structure-activity relationship of imidazo[1,2-a]benzimidazoles as corticotropin-releasing factor 1 receptor antagonists.](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Han X., Pin S.S., Burris K., Fung L.K., Huang S., Taber M.T., Zhang J., Dubowchik G.M.
Bioorganic and Medicinal Chemistry Letters,
2005
.

Begunov R.S., Fakhrutdinov A.N., Sokolov A.A.
ChemistrySelect,
2020
.
![Co-synthesis of 1-(3-aminopyridin-2-yl)-1H-benzimidazole and 3-(2-aminophenyl)-3H-imidazo[4,5-b]pyridine](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Begunov R.S., Shebunina T.V., Buzina V.A., Fakhrutdinov A.N., Shashkov A.S.
Russian Chemical Bulletin,
2016
.
![Synthesis of benzo[4,5]imidazo[1,2-a]quinoxalines by I2-mediated sp3 C–H amination](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Chen W., Du Y., Wang M., Fang Y., Yu W., Chang J.
Organic Chemistry Frontiers,
2020
.
![Synthesis and anti-HIV-1 activity of 4,5,6,7-tetrahydro-5-methylimidazo[4,5,1-jk][1,4]benzodiazepin-2(1H)-one (TIBO) derivatives](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Kukla M.J., Breslin H.J., Pauwels R., Fedde C.L., Miranda M., Scott M.K., Sherrill R.G., Raeymaekers A., Van Gelder J.
Journal of Medicinal Chemistry,
1991
.
![Synthesis and anti-HIV-1 activity of 4,5,6,7-tetrahydro-5-methylimidazo[4,5,1-jk][1,4]benzodiazepin-2(1H)-one (TIBO) derivatives. 2](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Kukla M.J., Breslin H.J., Diamond C.J., Grous P.P., Ho C.Y., Miranda M., Rodgers J.D., Sherrill R.G., De Clercq E., Pauwels R., Andries K., Moens L.J., Janssen M.A., Janssen P.A.
Journal of Medicinal Chemistry,
1991
.
![Synthesis and Anti-HIV-1 Activity of 4,5,6,7-Tetrahydro-5-methylimidazo[4,5,1-jk][1,4]benzodiazepin-2(1H)-one (TIBO) Derivatives. 3](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Breslin H.J., Kukla M.J., Ludovici D.W., Mohrbacher R., Ho W., Miranda M., Rodgers J.D., Hitchens T.K., Leo G.
Journal of Medicinal Chemistry,
1995
.
![Synthesis and Anti-HIV-1 Activity of 4,5,6,7-Tetrahydro-5-methylimidazo[4,5,1-jk][1,4]benzodiazepin-2(1H)-one (TlBO) Derivatives. 4](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Ho W., Kukla M.J., Breslin H.J., Ludovici D.W., Grous P.P., Diamond C.J., Miranda M., Rodgers J.D., Ho C.Y.
Journal of Medicinal Chemistry,
1995
.
![Synthesis and properties of poly[2-(4′-oxyphenylene)-5-benzimidazole] and a proton-exchange membrane produced on its basis](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Leikin A.Y., Rusanov A.L., Begunov R.S., Fomenkov A.I.
Polymer Science - Series C,
2009
.

Begunov R.S., Ryzvanovich G.A.
Chemistry of Heterocyclic Compounds,
2004
.
![Synthesis and biological evaluation of imidazolo[2,1-b]benzothiazole derivatives, as potential p53 inhibitors](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Christodoulou M.S., Colombo F., Passarella D., Ieronimo G., Zuco V., De Cesare M., Zunino F.
Bioorganic and Medicinal Chemistry,
2011
.

Subramanian P., Kaliappan K.P.
European Journal of Organic Chemistry,
2014
.

Zghaib Z., Guichou J., Vappiani J., Bec N., Hadj-Kaddour K., Vincent L., Paniagua-Gayraud S., Larroque C., Moarbess G., Cuq P., Kassab I., Deleuze-Masquéfa C., Diab-Assaf M., Bonnet P.
Bioorganic and Medicinal Chemistry,
2016
.
Begunov R.S., Shebunina T.V., Yakovleva Y.S., Firgang S.I.
Mendeleev Communications,
2013
.
Sochnev V.S., Kuz'menko T.A., Morkovnik A.S., Divaeva L.N., Podobina A.S., Zubenko A.A., Chepurnoy P.B., Borodkin G.S., Klimenko A.I.
Mendeleev Communications,
2021
.

Saliba J., Deleuze-Masquéfa C., Iskandarani A., El Eit R., Hmadi R., Mahon F., Bazarbachi A., Bonnet P., Nasr R.
Anti-Cancer Drugs,
2014
.
Begunov R.S., Aleksandrova Y.R., Yandulova E.Y., Nikolaeva N.S., Neganova M.E.
Mendeleev Communications,
2023