Keywords
chiral dioxane
cyrene
diol.
enolization
formaldehyde
levoglucosenone
Abstract
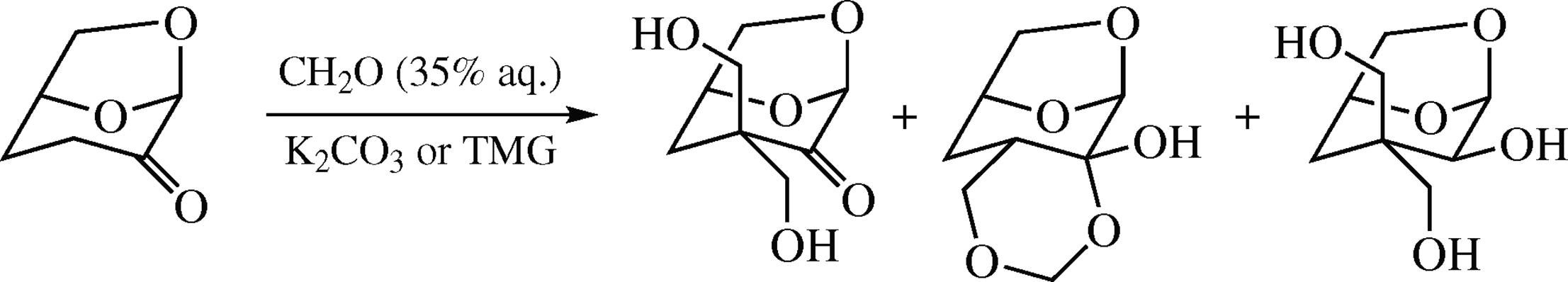
Cyrene reacts with 35% aqueous formaldehyde in the presence of K2CO3, tetramethylguanidine and Et3N to give three products, viz., major α,α-bis(hydroxymethyl) derivative, a product of its keto group reduction, and 3,5,11,12-tetraoxatricyclo[7.2.1.02,7]dodecan-2-ol. The options for the derivatization of the hydroxy groups with benzyl, tosyl, isopropylidene, and methylene acetal protecting groups were studied.
References
.

Stork G., D'Angelo J.
Journal of the American Chemical Society,
1974
.

Benkovic S.J., Fedor J.M.
Journal of the American Chemical Society,
1972
.

Stork G., Isobe M.
Journal of the American Chemical Society,
1975
.

Stork G., Isobe M.
Journal of the American Chemical Society,
1975
.

Grieco P.A., Hiroi K.
Journal of the Chemical Society Chemical Communications,
1972
.

Barton D.H., Motherwell W.B., Zard S.Z.
Journal of the Chemical Society Chemical Communications,
1982
.

Guiso M., Procaccio C., Fizzano M.R., Piccioni F.
Tetrahedron Letters,
1997
.
Sharipov B.T., Davydova A.N., Faizullina L.K., Valeev F.A.
Mendeleev Communications,
2019
.

Ledingham E.T., Stockton K.P., Greatrex B.W.
Australian Journal of Chemistry,
2017
.

Hossain N., van Halbeek H., De Clercq E., Herdewijn P.
Tetrahedron,
1998
.
Kh. Faizullina L., Khalilova Y.A., Sh. Karamysheva L., Salikhov S.M., Valeev F.A.
Mendeleev Communications,
2022