Keywords
carbon dioxide
gas hydrate
induction time.
nucleation
pH effect
Abstract
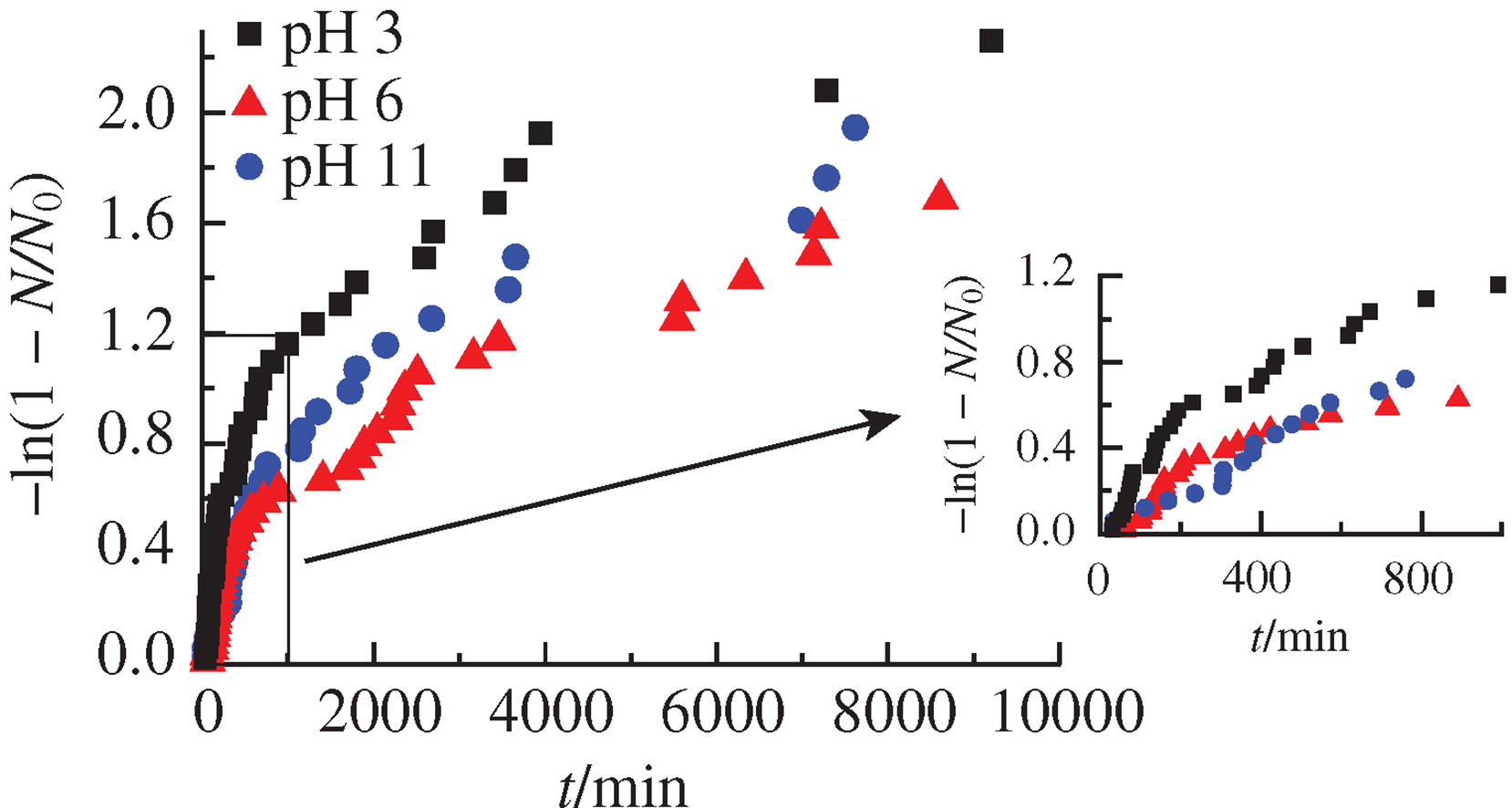
It has been shown that in the carbon dioxide-water system, the rate of gas hydrate nucleation increases with decreasing pH of the aqueous phase. Thus, a new approach has been demonstrated to control the rate of nucleation of carbon dioxide hydrate. Of interest is the question of the prospects for extending this approach to control the nucleation of other hydrates.
References
.

Meyssami B., Balaban M.O., Teixeira A.A.
Biotechnology Progress,
1992
.

Zhang J.S., Lo C., Somasundaran P., Lu S., Couzis A., Lee J.W.
Journal of Physical Chemistry C,
2008
.

Sagidullin A., Skiba S., Adamova T., Stoporev A., Strukov D., Kartopol’cev S., Manakov A.
ACS Sustainable Chemistry and Engineering,
2021
.

Vlasov V.A., Nesterov A.N., Reshetnikov A.M.
Russian Journal of Physical Chemistry A,
2020
.

Babu P., Linga P., Kumar R., Englezos P.
Energy,
2015
.

Ke W., Svartaas T.M., Chen D.
Journal of Natural Gas Science and Engineering,
2019
.

Shestakov V.A., Sagidullin A.K., Stoporev A.S., Grachev E.V., Manakov A.Y.
Journal of Molecular Liquids,
2020
.

Kashchiev D., Firoozabadi A.
Journal of Crystal Growth,
2002
.

Lal B., Nashed O.
Green Energy and Technology,
2020
.

Montazeri S.M., Kolliopoulos G.
Desalination,
2022
.
Stoporev A.S., Yu. Manakov A.
Mendeleev Communications,
2022
.
Adamova T.P., Strukov D.A., Yu. Manakov A., Nesterov A.N.
Mendeleev Communications,
2022