Keywords
Abstract
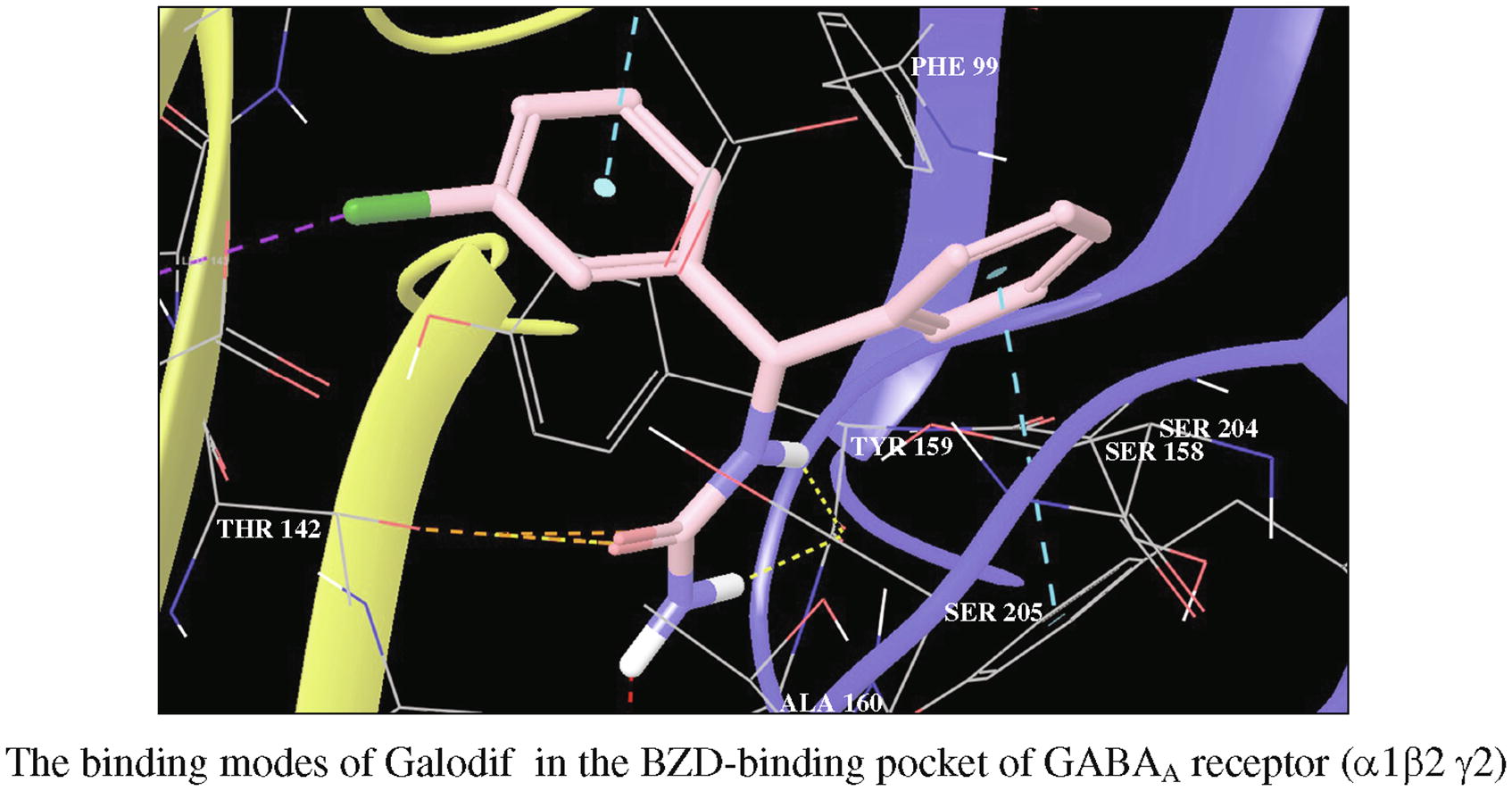
It has been experimentally established that the original new generation anticonvulsant Galodif, N-[(3-chlorophenyl)-(phenyl)methyl]urea, allosterically modulates GABAA receptor (GABAAR). Binding of [3H]flunitrazepam and [3H]Ro5-4864 to the benzodiazepine (BZD) site of GABAAR in the brain of Galodif-treated rats showes an increase in receptor affinity in Scatchard Plot for Ligand Receptor binding analysis. The results of molecular docking (Schrodinger program Glide) reveal that the enantiomers of Galodif are complementary to the BZD binding site of GABAAR; binding energy of R-Galodif is lower than that of S-Galodif (scoring GScore being -11.14 and -10.7 kcal mol-1, respectively); R-Galodif interacts with key amino acids at the α1γ2 interface: Tyr159, Tyr209, H101 Phe77 with high model fit - dG of insert: 7.41.
References