Keywords
1-halo-1-nitroethenes
3-bromo-3-nitroacrylates
3-methyl-1-phenylpyrazol-5-one
Meldrum’s acid
nitrocyclopropane
spirocyclopropane.
Abstract
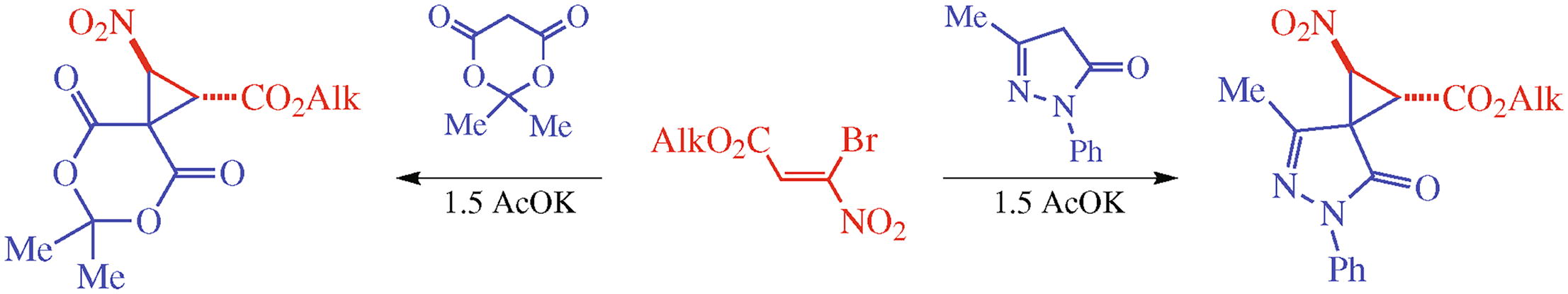
Reactions of alkyl 3-bromo-3-nitroacrylates with cyclic CH-acids, Meldrum’s acid or 3-methyl-1-phenylpyrazol-5-one, afford new spiro-fused 2-nitrocyclopropanecarboxylates. In the case of Meldrum’s acid, the products are formed as individual diastereomers. The obtained experimental results are confirmed by quantum chemical calculations (B3LYP/6-311+G(d,p) taking into account solvent effects).
References
.

Kuritsyna M.A., Pelipko V.V., Kataeva O.N., Baichurin R.I., Sadikov K.D., Smirnov A.S., Makarenko S.V.
Russian Chemical Bulletin,
2021
.
![Optimization of the Synthesis of Benzo[b]furan-3-carboxylates Based on Alkyl 3-Bromo-3-nitroacrylates](/storage/images/resized/oZgeErrVFhuDksyqFURLvYS1wtVSBWczh001igGo_small_thumb.webp)
Pelipko V.V., Baichurin R.I., Kondrashov E.V., Makarenko S.V.
Russian Journal of General Chemistry,
2021
.
Pelipko V.V., Baichurin R.I., Lyssenko K.A., Dotsenko V.V., Makarenko S.V.
Mendeleev Communications,
2022
.

Tomilov Y.V., Menchikov L.G., Novikov R.A., Ivanova O.A., Trushkov I.V.
Russian Chemical Reviews,
2018
.

Gharpure S.J., Nanda L.N.
Tetrahedron Letters,
2017
.

Selvi T., Srinivasan K.
Journal of Organic Chemistry,
2014
.

Russo A., Lattanzi A.
Tetrahedron Asymmetry,
2010
.

Lee H.J., Kim S.M., Kim D.Y.
Tetrahedron Letters,
2012
.

Gnad F., Reiser O.
Chemical Reviews,
2003
.

Achmatowicz M., Thiel O.R., Gorins G., Goldstein C., Affouard C., Jensen R., Larsen R.D.
Journal of Organic Chemistry,
2008
.

Fillion E., Carret S., Mercier L.G., Trépanier V.É.
Organic Letters,
2008
.

Zhao B., Du D.
European Journal of Organic Chemistry,
2015
.

Kryshtal G.V., Zhdankina G.M., Shashkov A.S., Zlotin S.G.
Russian Chemical Bulletin,
2011
.

Pekki A.I., Makarenko S.V., Altukhov K.V., Berestovitskaya V.M.
Russian Journal of General Chemistry,
2010
.

Smirnov A.S., Makarenko S.V., Berestovitskaya V.M., Pekki A.I., Konovalenko K.S.
Russian Journal of Organic Chemistry,
2006
.

Yashin N.V., Chmovzh T.N., Averina E.B., Kuznetsova T.S., Zefirov N.S.
Review Journal of Chemistry,
2014
.
![“On-water” one-pot four-component synthesis of novel 1H-furo[2,3-c]pyrazole-4-amine derivatives](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Noruzian F., Olyaei A., Hajinasiri R.
Research on Chemical Intermediates,
2019
.

Elinson M.N., Dorofeeva E.O., Vereshchagin A.N., Korshunov A.D., Egorov M.P.
Research on Chemical Intermediates,
2015
.

Donaldson W.A.
Tetrahedron,
2001
.

H. Stammer C.
Tetrahedron,
1990
.

Salaün J.
Topics in Current Chemistry,
1999
.

Pesciaioli F., Righi P., Mazzanti A., Bartoli G., Bencivenni G.
Chemistry - A European Journal,
2011
.

Dou X., Lu Y.
Chemistry - A European Journal,
2012
.

Roy S., Chen K.
Journal of the Chinese Chemical Society,
2013
.

Zlatopolskiy B.D., Radzom M., Zeeck A., de Meijere A.
European Journal of Organic Chemistry,
2006
.

Rapi Z., Démuth B., Keglevich G., Grűn A., Drahos L., Sóti P.L., Bakó P.
Tetrahedron Asymmetry,
2014
.

Keita M., De Bona R., Santos M.D., Lequin O., Ongeri S., Milcent T., Crousse B.
Tetrahedron,
2013
.
![An efficient and highly stereoselective synthesis of novel trifluoromethylated trans-dihydrofuro[2,3-c]pyrazoles using arsonium ylides](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Zhang J., Yang S., Zhang K., Chen J., Deng H., Shao M., Zhang H., Cao W.
Tetrahedron,
2012
.

Grygorenko O.O., Artamonov O.S., Komarov I.V., Mykhailiuk P.K.
Tetrahedron,
2011
.
![Synthesis and antimicrobial activity of some new 4-hetarylpyrazole and furo[2,3-c]pyrazole derivatives](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Bondock S., Khalifa W., Fadda A.A.
European Journal of Medicinal Chemistry,
2011
.

Vereshchagin A.N., Elinson M.N., Korshunov A.D., Korolev V.A., Egorov M.P.
Heterocyclic Communications,
2015
.

Hinton T., Chebib M., Johnston G.A.
Bioorganic and Medicinal Chemistry Letters,
2008
.

Jubault P., Pons A., Poisson T., Pannecoucke X., Charette A.
Synthesis,
2016
.

Averina E.B., Yashin N.V., Kuznetsova T.S., Zefirov N.S.
Russian Chemical Reviews,
2009
.

Salaon J., Baird M.S.
Current Medicinal Chemistry,
2022
.
Gomonov K.A., Pelipko V.V., Litvinov I.A., Baichurin R.I., Makarenko S.V.
Mendeleev Communications,
2023