Keywords
2-c]pyran-2
2-c]pyrans
4
5’-pyrimidine]
6-triones.
base-oxidant system
Cyclization
furo[3
morpholinium salts
N-bromosuccinimide
pyranones
pyrimidine-2
sodium acetate
spiro[furo- [3
Abstract
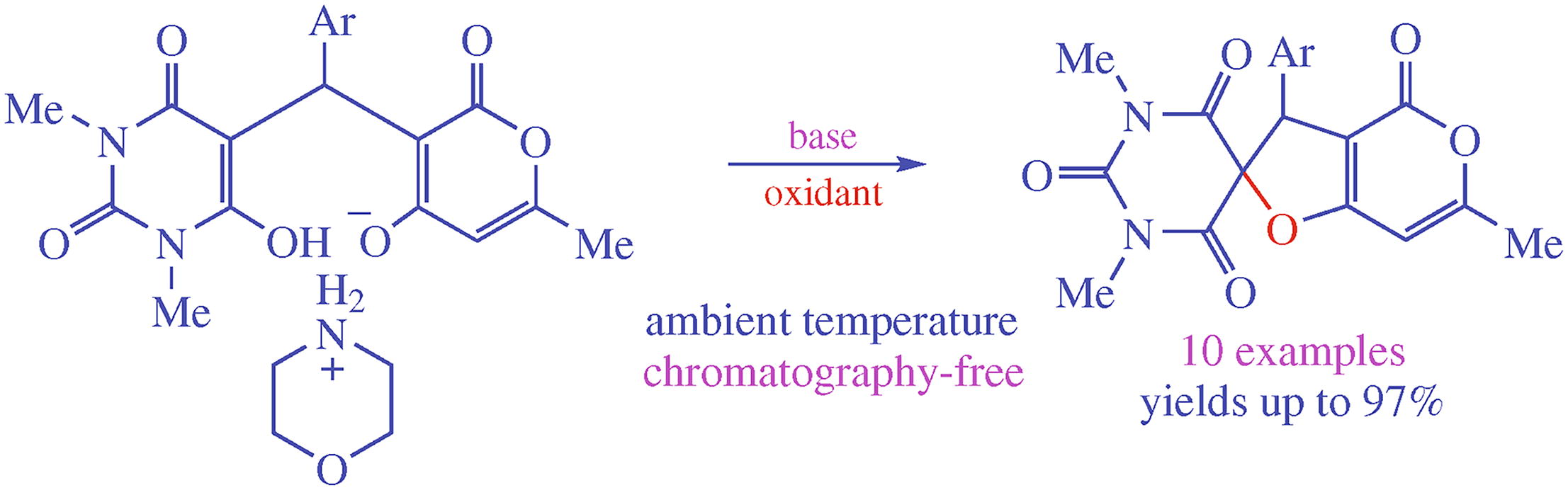
Spirocyclization of morpholinium 3-[(aryl)(1,3-dimethyl-2,4,6-trioxohexahydropyrimidin-5-yl)methyl]-6-methyl-2-oxo-2H-pyran-4-olate by the action of sodium acetate-N-bromosuccinimide system in ethanol at room temperature results in spiro[furo[3,2-c]pyran-2,5’-pyrimidine] derivatives in 92-98% yields, the protocol allowing to avoid column chromatography purification. This new highly efficient and facile procedure is a convenient way to substituted unsymmetrical spiro scaffold containing pyrimidine-2,4,6-trione and 2,3-dihydro-4H-furo[3,2-c]pyran-4-one fragments promising for biomedical applications.
References
.
![Cascade assembly of N,N′-dialkylbarbituric acids and aldehydes: a simple and efficient one-pot approach to the substituted 1,5-dihydro-2H,2′H-spiro(furo[2,3-d]pyrimidine-6,5′-pyrimidine)-2,2′,4,4′,6′(1′H,3H,3′H)-pentone framework](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Elinson M.N., Vereshchagin A.N., Stepanov N.O., Belyakov P.A., Nikishin G.I.
Tetrahedron Letters,
2010
.
![Electrocatalytic and chemical assembling of N,N′-dialkylbarbituric acids and aldehydes: efficient cascade approach to the spiro-[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′-(1′H,3H,3′H)-pentone framework](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Vereshchagin A.N., Elinson M.N., Dorofeeva E.O., Zaimovskaya T.A., Stepanov N.O., Gorbunov S.V., Belyakov P.A., Nikishin G.I.
Tetrahedron,
2012
.

Saikia I., Borah A.J., Phukan P.
Chemical Reviews,
2016
.

Hiesinger K., Dar’in D., Proschak E., Krasavin M.
Journal of Medicinal Chemistry,
2020
.

Pyrimidine-2,4,6-Triones: A New Effective and Selective Class of Matrix Metalloproteinase Inhibitors
Grams F., Brandstetter H., DAlò S., Geppert D., Krell H., Leinert H., Livi V., Menta E., Oliva A., Zimmermann G.
Biological Chemistry,
2001
.

Galati E.M., Monforte M.T., Miceli N., Raneri E.
Il Farmaco,
2001
.

Elinson M.N., Feducovich S.K., Stepanov N.O., Vereshchagin A.N., Nikishin G.I.
Tetrahedron,
2008
.

Fraser W., Suckling C.J., Wood H.C.
Journal of the Chemical Society Perkin Transactions 1,
1990
.

Zheng Y., Tice C.M., Singh S.B.
Bioorganic and Medicinal Chemistry Letters,
2014
.

Elinson M.N., Dorofeeva E.O., Vereshchagin A.N., Nikishin G.I.
Russian Chemical Reviews,
2015
.

Schneider P., Schneider G.
Angewandte Chemie - International Edition,
2017
.

Elinson M.N., Vereshchagin A.N., Feducovich S.K., Zaimovskaya T.A., Starikova Z.A., Belyakov P.A., Nikishin G.I.
Tetrahedron Letters,
2007
.

Mphahlele M.J.
Molecules,
2009
.
![Multicomponent Electrocatalytic Selective Approach to Unsymmetrical Spiro[furo[3,2-c]pyran-2,5′-pyrimidine] Scaffold under a Column Chromatography-Free Protocol at Room Temperature](/storage/images/resized/MjH1ITP7lMYGxeqUZfkt2BnVLgjkk413jwBV97XX_small_thumb.webp)
Ryzhkova Y.E., Elinson M.N., Vereshchagin A.N., Karpenko K.A., Ryzhkov F.V., Ushakov I.E., Egorov M.P.
Chemistry,
2022
.

Wei Y., Lin S., Xue H., Liang F., Zhao B.
Organic Letters,
2012
.

Huang C., Zeng Y., Cheng H., Hu A., Liu L., Xiao Y., Zhang J.
Organic Letters,
2017
.
![Synthesis of Functionalized Benzo[g]indoles and 1-Naphthols via Carbon–Carbon Triple Bond Breaking/Rearranging](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Wang J., Zhou P., Li G., Hao W., Tu S., Jiang B.
Organic Letters,
2017
.

McGlacken G.P., Fairlamb I.J.
Natural Product Reports,
2005
.

Wang B., Wong H.N.
Bulletin of the Chemical Society of Japan,
2018
.

.

Kim S., Pudzianowski A.T., Leavitt K.J., Barbosa J., McDonnell P.A., Metzler W.J., Rankin B.M., Liu R., Vaccaro W., Pitts W.
Bioorganic and Medicinal Chemistry Letters,
2005
.

Zheng Y., Tice C.M.
Expert Opinion on Drug Discovery,
2016
.

Cleary T., Rawalpally T., Kennedy N., Chavez F.
Tetrahedron Letters,
2010
.

Elinson M.N., Vereshchagin A.N., Stepanov N.O., Ilovaisky A.I., Vorontsov A.Y., Nikishin G.I.
Tetrahedron,
2009
.
Vereshchagin A.N., Elinson M.N., Stepanov N.O., Nikishin G.I.
Mendeleev Communications,
2009
.
![An expedient, regioselective synthesis of 2-alkylamino- and 2-alkylthiothiazolo[5,4-e]indoles](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Chakrabarty M., Kundu T., Arima S., Harigaya Y.
Tetrahedron Letters,
2005
.

Vara Prasad J.V., Pavlovsky A., Para K.S., Ellsworth E.L., Tummino P.J., Nouhan C., Ferguson D.
Bioorganic and Medicinal Chemistry Letters,
1996
.
Yin P., Liu X., Qiu Y., Cai J., Qin J., Zhu H., Li Q.
Asian Pacific Journal of Cancer Prevention,
2012
.

Katsamakas S., Papadopoulos A.G., Kouskoura M.G., Markopoulou C.K., Hadjipavlou-Litina D.
Future Medicinal Chemistry,
2019