Keywords
[3 + 2] dipolar cycloaddition
1
2
3-thiadiazoles.
Chan–Evans–Lam arylation
Lawesson’ reagent
methyl propiolate
pyrazoles
α-acetyl-α-diazomethane sulfonamide
Abstract
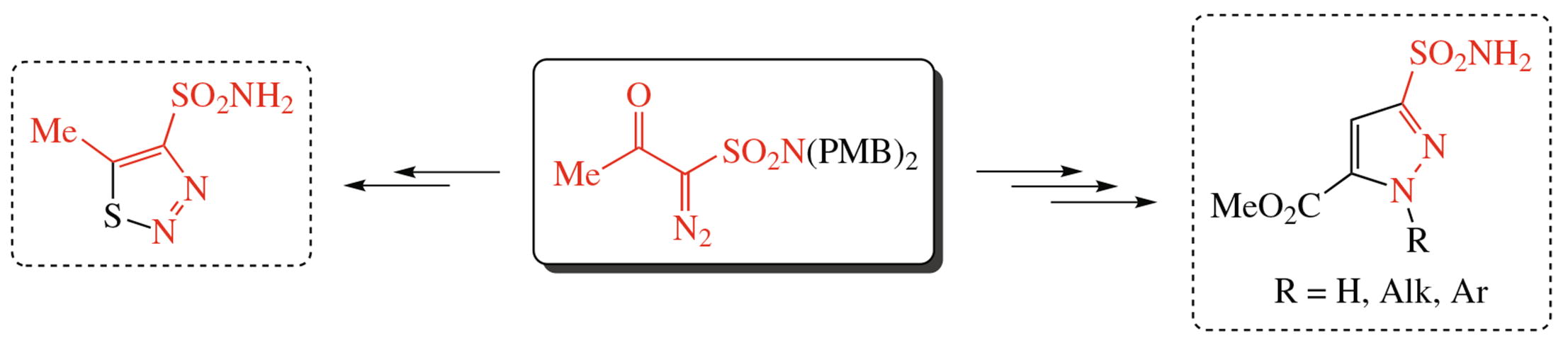
An N,N-bis(p-methoxybenzyl)-protected α-acetyl-α-diazo-methane sulfonamide proved to be a useful building block for accessing new 5-methyl-1,2,3-thiadiazole-4-sulfonamide as well as methyl 3-sulfamoyl-1H-pyrazole-5-carboxylate. The latter was further subjected to N-alkylation and N-arylation reactions. All resulting compounds showed potent inhibition of I, II and particularly of cancer-related IX and XII isoforms of human carbonic anhydrase.
References
.

Dar’in D., Kantin G., Krasavin M.
Chemical Communications,
2019
.
Paramonova P., Sharonova T., Kalinin S., Chupakhin E., Bunev A., Krasavin M.
Mendeleev Communications,
2022
.

Alterio V., Di Fiore A., D’Ambrosio K., Supuran C.T., De Simone G.
Chemical Reviews,
2012
.

Dar’in D., Krasavin M.
Journal of Organic Chemistry,
2016
.

Supuran C.T.
Nature Reviews Drug Discovery,
2008
.

Fleming J.J., Du Bois J.
Journal of the American Chemical Society,
2006
.

Fleming J.J., McReynolds M.D., Du Bois J.
Journal of the American Chemical Society,
2007
.

Tanpure R.P., Ren B., Peat T.S., Bornaghi L.F., Vullo D., Supuran C.T., Poulsen S.
Journal of Medicinal Chemistry,
2015
.

Bubyrev A., Adamchik M., Dar’in D., Kantin G., Krasavin M.
Journal of Organic Chemistry,
2021
.

Bubyrev A., Malkova K., Kantin G., Dar’in D., Krasavin M.
Journal of Organic Chemistry,
2021
.

Berrino E., Supuran C.T.
Expert Opinion on Drug Discovery,
2019
.

Wang Y., Guo H., Tang G., He Q., Zhang Y., Hu Y., Wang Y., Lin Z.
Computational Biology and Chemistry,
2019