Keywords
5-nitrofuroyl.
azetidines
instability in deprotected form
privileged scaffold
pyrimidines
spirocycles
Abstract
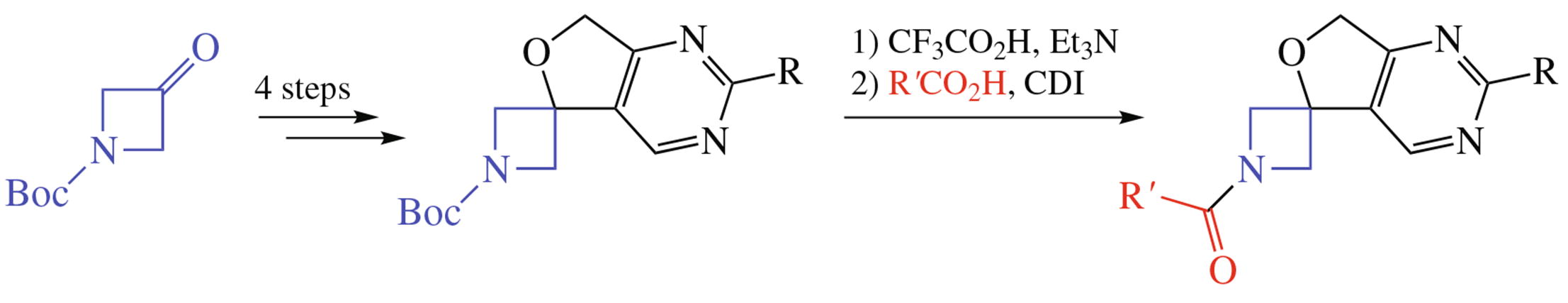
A novel spirocyclic scaffold of 7'H-spiro[azetidine-3,5'-furo[3,4-d]pyrimidine] chemotype was synthesized in N-Boc-protected form. However, the scaffold was revealed to be unstable to storage when deprotected. The solution was found in the brief removal of the Boc protecting group and rapid acylation of the liberated NH-azetidine with a carboxylic acid imidazolide.
References
.

Hiesinger K., Dar’in D., Proschak E., Krasavin M.
Journal of Medicinal Chemistry,
2020
.

Chupakhin E., Babich O., Prosekov A., Asyakina L., Krasavin M.
Molecules,
2019
.

Raymer B., Bhattacharya S.K.
Journal of Medicinal Chemistry,
2018
.

Welsch M.E., Snyder S.A., Stockwell B.R.
Current Opinion in Chemical Biology,
2010
.

Eriksson B.I., Carlsson S., Halvarsson M., Risberg B., Mattsson C.
Thrombosis and Haemostasis,
1997
.

Moffat J.G., Vincent F., Lee J.A., Eder J., Prunotto M.
Nature Reviews Drug Discovery,
2017
.

Nadin A., Hattotuwagama C., Churcher I.
Angewandte Chemie - International Edition,
2012
.
Krasavin M., Shetnev A., Panova V., Ivanovskyi S., Kalinin S., Vinogradova T., Sharoyko V., Yablonsky P.
Mendeleev Communications,
2022
.
![Construction of Multifunctional Modules for Drug Discovery: Synthesis of Novel Thia/Oxa-Azaspiro[3.4]octanes](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Li D.B., Rogers-Evans M., Carreira E.M.
Organic Letters,
2013
.

Butler C.R., Beck E.M., Harris A., Huang Z., McAllister L.A., am Ende C.W., Fennell K., Foley T.L., Fonseca K., Hawrylik S.J., Johnson D.S., Knafels J.D., Mente S., Noell G.S., Pandit J., et. al.
Journal of Medicinal Chemistry,
2017
.

Lai C., Lo I., Hewage R.T., Chen Y., Chen C., Lee C., Lin S., Tang M., Lin H.
Angewandte Chemie - International Edition,
2017
.

Yan F., Auerbach D., Chai Y., Keller L., Tu Q., Hüttel S., Glemser A., Grab H.A., Bach T., Zhang Y., Müller R.
Angewandte Chemie - International Edition,
2018
.

Higuchi K., Suzuki K., Nakanishi H., Yamaguchi H., Nishizawa N., Mori S.
Plant Physiology,
1999
.

Lip G.Y., Rasmussen L.H., Olsson S.B., Jensen E.C., Persson A.L., Eriksson U., Wahlander K.F.
European Heart Journal,
2009
.
Verbitskiy E.V., Baskakova S.A., Gerasimova N.A., Evstigneeva N.P., Zil’berberg N.V., Kungurov N.V., Kravchenko M.A., Rusinov G.L., Chupakhina O.N., Charushin V.N.
Mendeleev Communications,
2018
.

OIZUMI K., NISHINO H., KOIKE H., SADA T., MIYAMOTO M., KIMURA T.
The Japanese Journal of Pharmacology,
1989