Keywords
hybrid halide perovskites
perovskite photovoltaics.
polyiodides
solid phase equilibria
solid solutions
Abstract
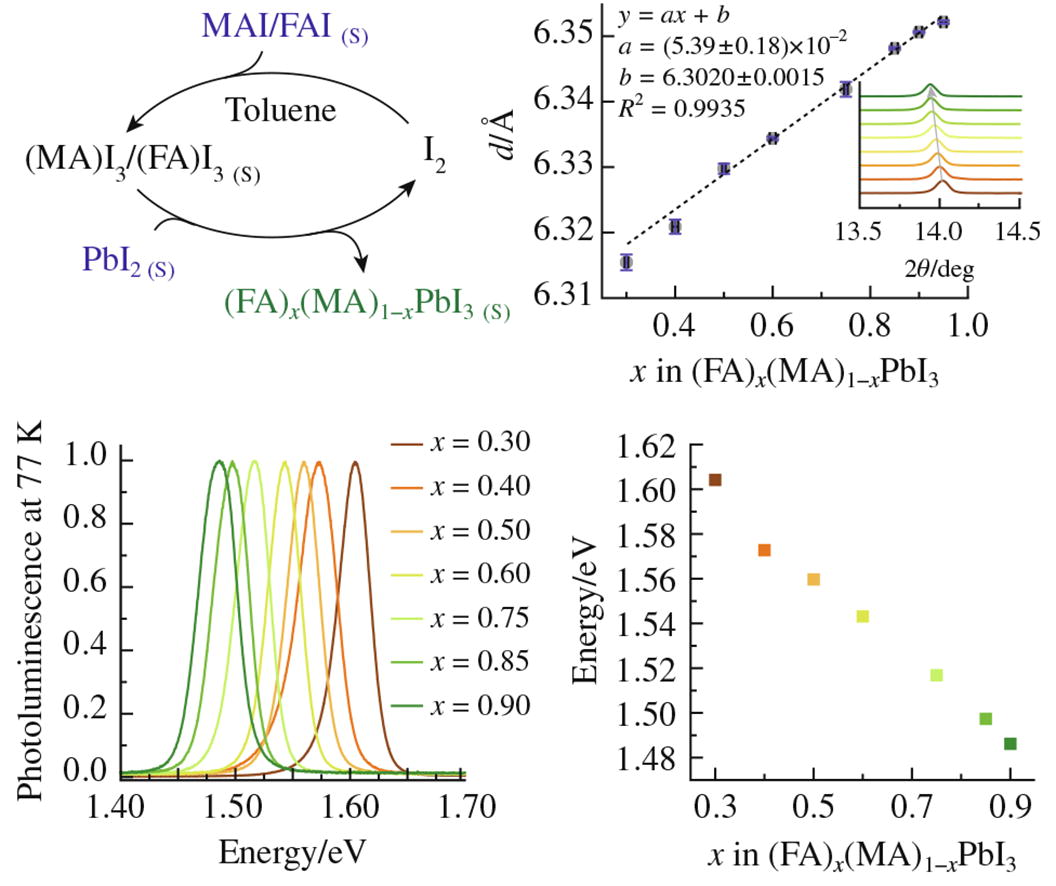
A new facile method for the synthesis of mixed-cation halide R2 = 0.9935 perovskites based on the chemical conversion of solid precursors (organic halides and lead halides) via an iodine-mediated transport reaction in inert liquid media under mild conditions is described. The equilibrium nature of the conversion provides an exact match between the stoichiometry of the resulting perovskite powder and the molar ratio of the precursors. This method can serve as a useful tool for the synthesis of complex perovskite precursors and the investigation of phase equilibria
References
.

Petříček V., Dušek M., Palatinus L.
Zeitschrift fur Kristallographie - Crystalline Materials,
2014
.

Katan C., Mercier N., Even J.
Chemical Reviews,
2019
.

Sutherland B.R., Sargent E.H.
Nature Photonics,
2016
.

De Wolf S., Holovsky J., Moon S., Löper P., Niesen B., Ledinsky M., Haug F., Yum J., Ballif C.
Journal of Physical Chemistry Letters,
2014
.

Tutantsev A.S., Udalova N.N., Fateev S.A., Petrov A.A., Chengyuan W., Maksimov E.G., Goodilin E.A., Tarasov A.B.
Journal of Physical Chemistry C,
2020
.

Park N., Zhu K.
Nature Reviews Materials,
2020
.

Dong Q., Fang Y., Shao Y., Mulligan P., Qiu J., Cao L., Huang J.
Science,
2015
.

Fu Y., Zhu H., Chen J., Hautzinger M.P., Zhu X.-., Jin S.
Nature Reviews Materials,
2019
.

Conings B., Drijkoningen J., Gauquelin N., Babayigit A., D'Haen J., D'Olieslaeger L., Ethirajan A., Verbeeck J., Manca J., Mosconi E., Angelis F.D., Boyen H.
Advanced Energy Materials,
2015
.

Petrov A.A., Sokolova I.P., Belich N.A., Peters G.S., Dorovatovskii P.V., Zubavichus Y.V., Khrustalev V.N., Petrov A.V., Grätzel M., Goodilin E.A., Tarasov A.B.
Journal of Physical Chemistry C,
2017
.

Pisanu A., Ferrara C., Quadrelli P., Guizzetti G., Patrini M., Milanese C., Tealdi C., Malavasi L.
Journal of Physical Chemistry C,
2017
.

Murali B., Kolli H.K., Yin J., Ketavath R., Bakr O.M., Mohammed O.F.
ACS Materials Letters,
2019
.

Van Gompel W.T., Herckens R., Reekmans G., Ruttens B., D’Haen J., Adriaensens P., Lutsen L., Vanderzande D.
Journal of Physical Chemistry C,
2018
.

Petrov A.A., Fateev S.A., Zubavichus Y.V., Dorovatovskii P.V., Khrustalev V.N., Zvereva I.A., Petrov A.V., Goodilin E.A., Tarasov A.B.
Journal of Physical Chemistry Letters,
2019
.

Grishko A.Y., Eliseev A.A., Goodilin E.A., Tarasov A.B.
Chemistry of Materials,
2020
.

Voronin O.S., Grishko A.Y., Finkelberg Y.M., Petrov A.A., Goodilin E.A., Tarasov A.B.
Journal of Physical Chemistry Letters,
2022
.

Udalova N.N., Tutantsev A.S., Fateev S.A., Zharenova E.A., Belich N.A., Nemygina E.M., Ryabova A.V., Goodilin E.A., Tarasov A.B.
Russian Journal of Inorganic Chemistry,
2021
.

Ruan S., Fan R., Pai N., Lu J., Webster N.A., Ruan Y., Cheng Y., McNeill C.R.
Chemical Communications,
2019
.

Jacobsson T.J., Hultqvist A., García-Fernández A., Anand A., Al-Ashouri A., Hagfeldt A., Crovetto A., Abate A., Ricciardulli A.G., Vijayan A., Kulkarni A., Anderson A.Y., Darwich B.P., Yang B., Coles B.L., et. al.
Nature Energy,
2021
.
Petrov A.A., Marchenko E.I., Fateev S.A., Yumao L., Goodilin E.A., Tarasov A.B.
Mendeleev Communications,
2022
.
Marchenko E.I., Fateev S.A., Ordinartsev A.A., Ivlev P.A., Goodilin E.A., Tarasov A.B.
Mendeleev Communications,
2022