Keywords
borothermal reduction
epoxy
high-temperature synthesis.
vanadium diboride
vanadium oxide
Abstract
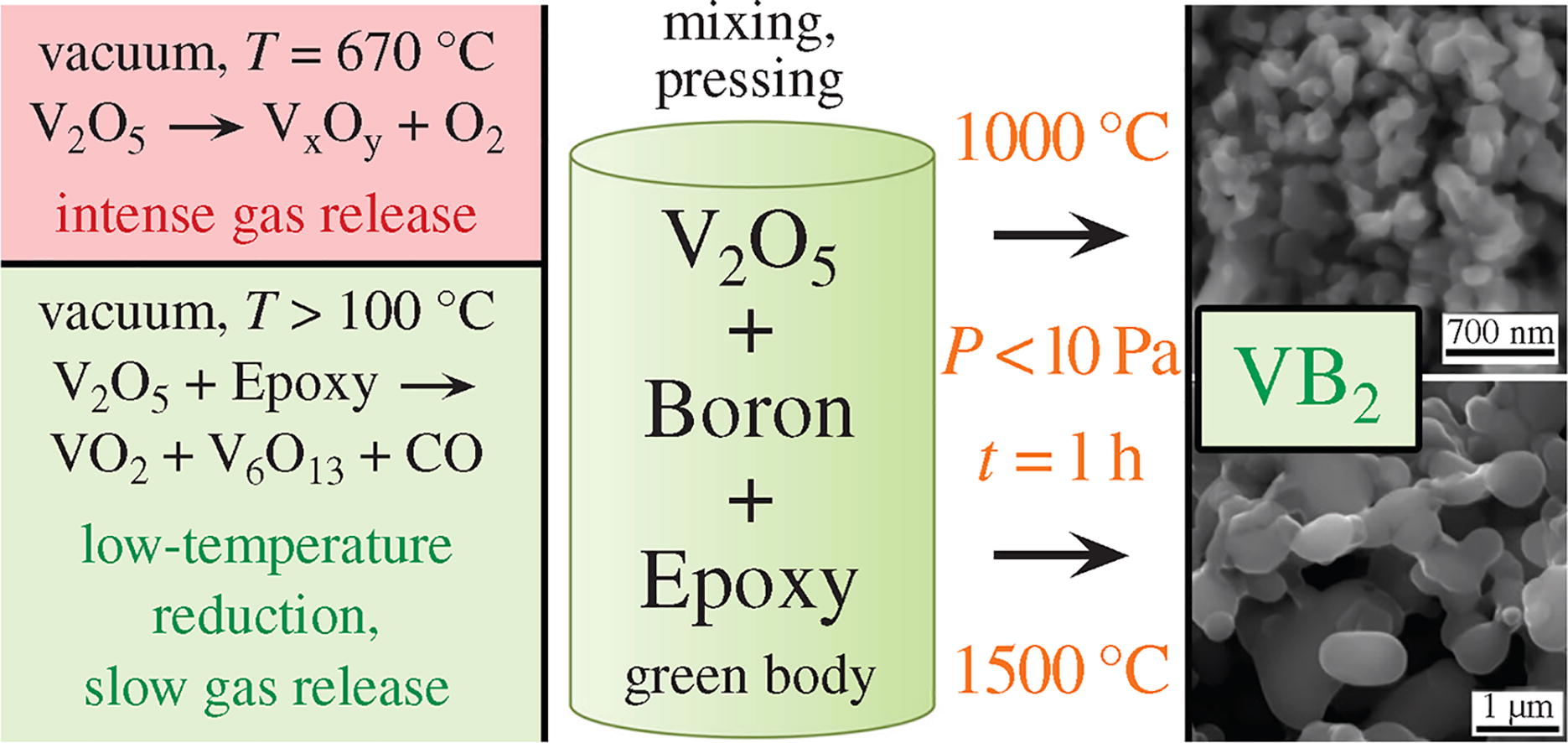
Vanadium diboride was directly synthesized by borothermal reduction of V2O5 with the addition of epoxy resin as a reducing agent for the low-temperature reduction of vanadium(V) to vanadium(IV), which leads to the gradual removal of oxygen by the formation of CO gas. The slow rate of gas release prevents destruction of green body, which usually occurs during conventional borothermal reduction. This makes it possible to directly obtain VB2 powder with an average particle size of 200-300 nm without need to prepare intermediate lower vanadium oxides.
References
.

Wang P., Kumar R., Sankaran E.M., Qi X., Zhang X., Popov D., Cornelius A.L., Li B., Zhao Y., Wang L.
Inorganic Chemistry,
2018
.

Gurin V.N.
Russian Chemical Reviews,
1972
.

Wang F., Wu M., Chowdari B.V., Wen Z.
ACS applied materials & interfaces,
2020
.

Qin G., Cui Q., Du A., Wang W., Sun Q.
ChemCatChem,
2019
.

Lee E., Park H., Joo H., Fokwa B.P.
Angewandte Chemie - International Edition,
2020
.

Korobov I.I., Kovalev D.Y., Kalinnikov G.V., Konovalikhin S.V., Khomenko N.Y., Vinokurov A.A., Ivanov A.V., Shilkin S.P.
Inorganic Materials,
2020
.

Low-Temperature Synthesis of VB2 Nanopowders by a Molten-Salt-Assisted Borothermal Reduction Process
Wu Y., Zhang G., Wang Y., Xu R.
Metallurgical and Materials Transactions B,
2019
.

Rao L., Gillan E.G., Kaner R.B.
Journal of Materials Research,
1995
.

KIEFFER R., BENESOVSKY F.
Powder Metallurgy,
1958
.

de Castro M.S., Ferreira C.L., de Avillez R.R.
Infrared Physics and Technology,
2013
.

Wu Y., Zhang G., Wang Y., Xu R., Chou K.
Ceramics International,
2019
.

Tian L., Wang F., Zhang Z., Min S.
International Journal of Hydrogen Energy,
2020
.

Wei Y., Huang Z., Zhou L., Ran S.
International Journal of Materials Research,
2015
.

Kim J.W., Shim J., Ahn J., Cho Y.W., Kim J., Oh K.H.
Materials Letters,
2008
.

Licht S., Hettige C., Lau J., Cubeta U., Wu H., Stuart J., Wang B.
Electrochemical and Solid-State Letters,
2011
.

Shibuya K., Sawa A.
AIP Advances,
2015
.

Shi L., Gu Y., Chen L., Yang Z., Ma J., Qian Y.
Materials Letters,
2004
.

Peshev P., Leyarovska L., Bliznakov G.
Journal of the Less Common Metals,
1968
.

Wang H., Stolyarova V.L., Lopatin S.I., Kutuzova M.E., Seetharaman S.
Rapid Communications in Mass Spectrometry,
2010
.

Lefler M., Stuart J., Parkey J., Licht S.
Journal of the Electrochemical Society,
2016
.

Licht S., Ghosh S., Wang B., Dianlu J., Hettige C., Lau J., Asercion J.
ECS Transactions,
2011
.

Ordanyan S.S., Vikhman S.V., Nesmelov D.D., Danilovich D.P., Panteleev I.B.
Advances in Science and Technology,
2014