Keywords
2
3-diaminonaphthalene
6-di-tert-butyl-p-benzoquinone
alloxan
dimedone
enamines
spiro compounds
sterically hindered phenols.
Abstract
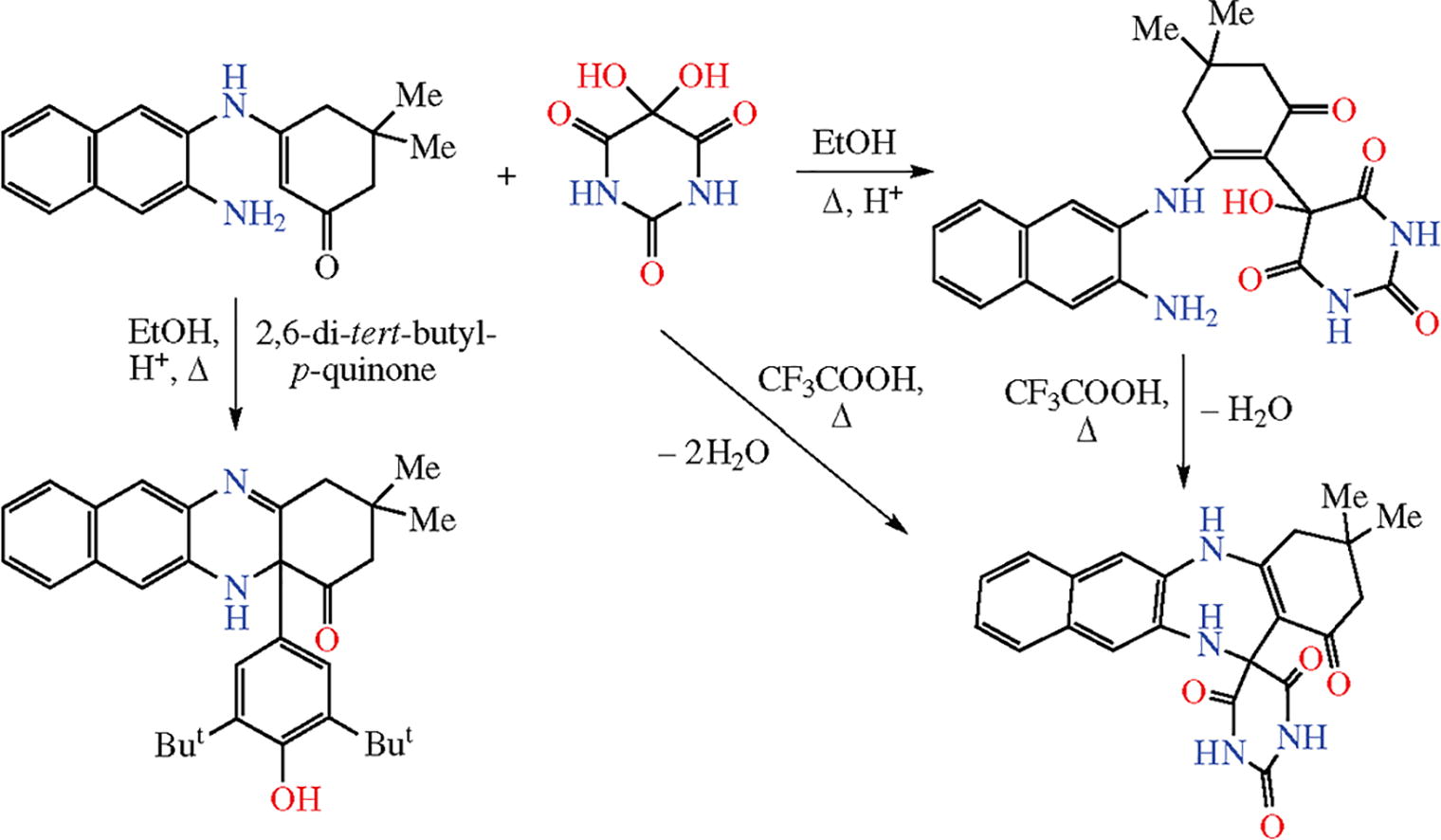
The reaction of 2,3-diaminonaphthalene with dimedone gave 3-[(3-amino-2-naphthyl)amino]-5,5-dimethylcyclohex-2-en-1-one that reacts with alloxan to afford a product of addition at the enamine moiety. The latter is converted to a spiro derivative on heating in CF3COOH, or undergoes condensation with 2,6-di-tert-butyl-p-benzoquinone to give a benzophenazinone derivative with a sterically hindered phenol substituent.
References
.
Ukhin L.Y., Suponitsky K.Y., Shepelenko E.N., Belousova L.V., Popova O.S., Borodkin G.S.
Mendeleev Communications,
2015
.
![Novel synthesis of oxonine derivatives from 3-[(2-aminophenyl)amino]-5,5-dimethyl-2-cyclohexene-1-one and o-quinones](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Ukhin L.Y., Suponitsky K.Y., Shepelenko E.N., Belousova L.V., Borodkin G.S.
Tetrahedron Letters,
2012
.

Todd P.A., Clissold S.P.
Drugs,
1990
.

Borodkin G.S., Zaichenko S.B., Borodkina I.G., Belousova L.V., Ukhin L.Y.
Russian Chemical Bulletin,
2015
.

Ukhin L.Y., Suponitskii K.Y., Shepelenko E.N., Belousova L.V., Orlova Z.I., Borodkin G.S.
Russian Chemical Bulletin,
2011
.

Meletiadis J., Chanock S., Walsh T.J.
Clinical Microbiology Reviews,
2006
.

Greenhill J.V.
Journal of the Chemical Society Perkin Transactions 1,
1976
.
![Synthesis of 1-substituted 3-anilino-4-diethylaminomethyl-5-oxo-3,4-dehydropiperidines and 2-substituted 1,2,3,5,6,11-hexahydro-5-phenyl-4H-pyrido[3,4-b][1,5]benzodiazepin-4-ones](/storage/images/resized/bRyGpdm98BkAUYiK1YFNpl5Z7hPu6Gd87gbIeuG3_small_thumb.webp)
Tamura Y., Chen L.C., Fujita M., Kita Y.
Journal of Heterocyclic Chemistry,
1980
.
Ukhin L.Y., Orlova Z.I., Suponitsky K.Y., Shepelenko E.N., Belousova L.V.
Mendeleev Communications,
2014
.

Datta B., Madhusudana Reddy M.B., Pasha M.A.
Synthetic Communications,
2011
.

Xue L., Jiang B., Tu M., Tu S.
Tetrahedron Letters,
2012
.

Hirata T., Narumiya S.
Advances in Immunology,
2012
.
Matushevskaya Y.V., Svirshchevskaya Y.V.
Vestnik dermatologii i venerologii,
2014
.
![Synthesis of 3, 3-Dimethyl-2, 3, 4, 5, 10, 11-hexahydro-11-phenyl-1H-dibenzo [b, e] [1, 4] diazepin-1-one, a New Tricyclic System](/storage/images/resized/KBGMujzjwr0rGFxg8Kz0qvYdFHkcBo5w0GTtfiiU_small_thumb.webp)
MIYANO S., ABE N.
Chemical and Pharmaceutical Bulletin,
2011