Keywords
2-diaminocyclohexane
anilines
catalysis
cytotoxic activity.
heterocyclization
hexaazadibenzotetracenes
polycyclic compounds
trans-1
Abstract
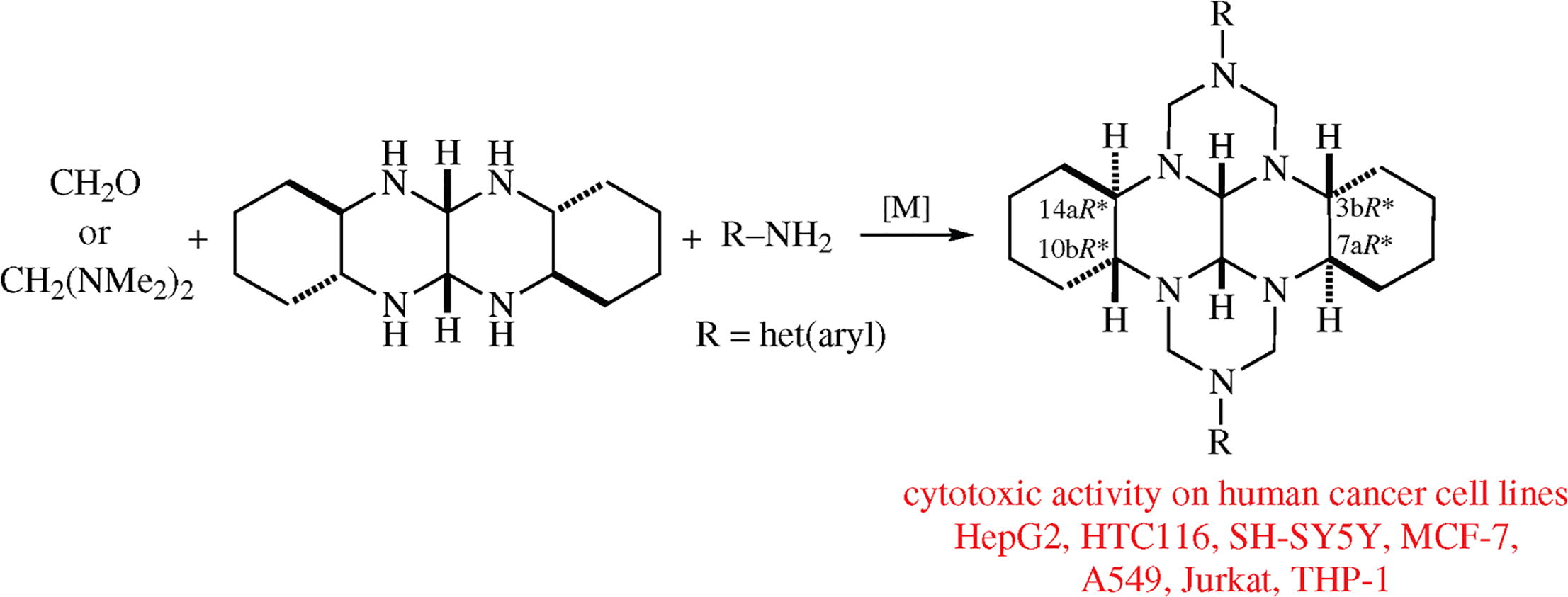
A one-pot synthesis of N,N'-disubstituted (3bR*,7aR*,10bR*,14aR*)-octadecahydro-1H ,8H-2,3a,7b,9,10a,14b-hexaazadibenzo[fg, op]tetracenes via the catalytic heterocyclization of trans-1,6,7,12-tetraaza-perhydrotetracene with formaldehyde or CH2(NMe2)2 and anilines has been accomplished. Preliminary screening of thus obtained perhydro hexaazadibenzotetracenes for cytotoxic activity has been performed.
References
.
Rakhimova E.B., Kirsanov V.Y., Mescheryakova E.S., Ibragimov A.G., Dzhemilev U.M.
Mendeleev Communications,
2020
.

Rakhimova E.B., Kirsanov V.Y., Ibragimov A.G.
Russian Journal of Organic Chemistry,
2022
.

Rakhimova E.B., Kirsanov V.Y., Ibragimov A.G., Dzhemilev U.M.
Russian Journal of Organic Chemistry,
2018
.

Rakhimova E.B., Kirsanov V.Y., Tret'yakova E.V., Khalilov L.M., Ibragimov A.G., Dzhemileva L.U., D'yakonov V.A., Dzhemilev U.M.
RSC Advances,
2020
.
![Synthesis, Structure, and Biological Activity of 2,6-Disubstituted 2,3a,4a,6,7a,8a-Hexaazaperhydrocyclopenta[ def ]fluorene-4-thioxo-8-ones](/storage/images/resized/xqixcltwJYe6H8Uco2JbAFfIOzt7UNKH0OcPOPzO_small_thumb.webp)
Barsegyan Y., Baranov V., Kravchenko A., Strelenko Y., Anikina L., Karnoukhova V., Kolotyrkina N.
Synthesis,
2018
.

Pearson R.G.
Journal of Chemical Education,
1968
.

Kucherenko A.S., Kostenko A.A., Komogortsev A.N., Lichitsky B.V., Fedotov M.Y., Zlotin S.G.
Journal of Organic Chemistry,
2019
.

Dvornikova I.A., Buravlev E.V., Frolova L.L., Nelyubina Y.V., Chukicheva I.Y., Kuchin A.V.
Russian Journal of Organic Chemistry,
2011
.

Evans D.A., Mito S., Seidel D.
Journal of the American Chemical Society,
2007
.
![Ni(II)−Bis[(R,R)-N,N‘-dibenzylcyclohexane-1,2-diamine]Br2 Catalyzed Enantioselective Michael Additions of 1,3-Dicarbonyl Compounds to Conjugated Nitroalkenes](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Evans D.A., Seidel D.
Journal of the American Chemical Society,
2005
.

Omer K.H., Seliman A.A., Altaf M., Casagrande N., Aldinucci D., Altuwaijri S., Isab A.A.
Polyhedron,
2015
.

Khokhar A.R., Al-Baker S., Shamsuddin S., Siddik Z.H.
Journal of Medicinal Chemistry,
1997
.

Zaleska B., Socha R., Karelus M., Szneler E., Grochowski J., Serda P.
Journal of Organic Chemistry,
2003
.

Wojaczyńska E., Bąkowicz J., Dorsz M., Skarżewski J.
Journal of Organic Chemistry,
2013
.

Makhmudiyarova N.N., Prokof’ev K.I., Mudarisova L.V., Ibragimov A.G., Dzhemilev U.M.
Russian Journal of Organic Chemistry,
2013
.

Qiu R., Chen Y., Yin S., Xu X., Au C.
RSC Advances,
2012
.

Hopff S.M., Wang Q., Frias C., Ahrweiler M., Wilke N., Wilke N., Berkessel A., Prokop A.
Journal of Cancer Research and Clinical Oncology,
2021
.

Dragoun M., Günther T., Frias C., Berkessel A., Prokop A.
Journal of Cancer Research and Clinical Oncology,
2018
.
![New cyclic aminals derived from rac-trans-1,2-diaminocyclohexane: synthesis and crystal structure of racemic 1,8,10,12-tetraazatetracyclo[8.3.1.1.8,1202,7] pentadecane and a route to its enantiomerically pure (R,R) and (S,S) isomers](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Rivera A., Quiroga D., Jiménez-Cruz L., Fejfarová K., Dušek M.
Tetrahedron Letters,
2012
.

Berkessel A., Eröksüz S., Neudörfl J.
Synlett,
2017
.

Padmaja M., Periasamy M.
Tetrahedron Asymmetry,
2004
.

Iwanejko J., Wojaczyńska E., Trynda J., Maciejewska M., Wietrzyk J., Kochel A., Wojaczyński J.
Tetrahedron,
2017
.

Borisova N.E., Reshetova M.D., Ustynyuk Y.A.
Russian Chemical Reviews,
2007