Keywords
antibacterial activity.
antimycobacterial activity
epoxide hydrolase
membrane transporter Mmpl3
piperidines
spirocyclic compounds
ureas
Abstract
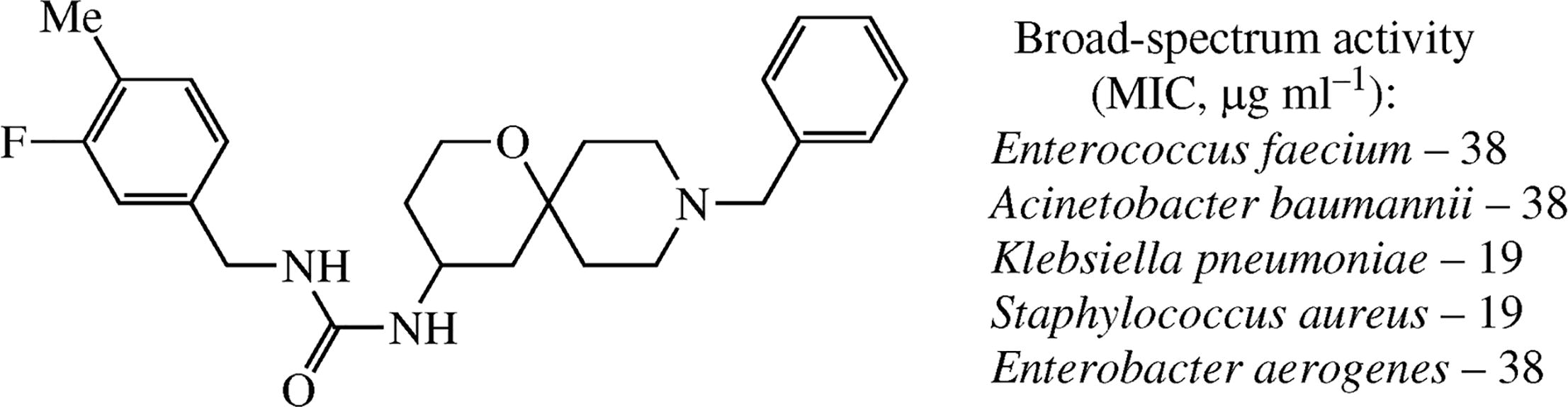
Antimycobacterial activity of certain ureas as well as spirocyclic piperidines described in the literature prompted us to synthesize and test a series of hybrids of spirocyclic piperidine with ureas. Surprisingly, no activity was detected against Mycobacterium tuberculosis. However, significant antibacterial activity was identified and confirmed against common gram-positive as well as gram-negative bacteria.
References
.

Krasavin M., Lukin A., Bagnyukova D., Zhurilo N., Golovanov A., Zozulya S., Zahanich I., Moore D., Tikhonova I.G.
European Journal of Medicinal Chemistry,
2017
.

Wiegand I., Hilpert K., Hancock R.E.
Nature Protocols,
2008
.

Welsch M.E., Snyder S.A., Stockwell B.R.
Current Opinion in Chemical Biology,
2010
.

Lukin A., Bagnyukova D., Kalinchenkova N., Zhurilo N., Krasavin M.
Tetrahedron Letters,
2016
.

Krasavin M., Lukin A., Bagnyukova D., Zhurilo N., Zahanich I., Zozulya S., Ihalainen J., Forsberg M.M., Lehtonen M., Rautio J., Moore D., Tikhonova I.G.
Bioorganic and Medicinal Chemistry,
2016
.

Krasavin M., Lukin A., Vedekhina T., Manicheva O., Dogonadze M., Vinogradova T., Zabolotnykh N., Rogacheva E., Kraeva L., Sharoyko V., Tennikova T.B., Dar'in D., Sokolovich E.
European Journal of Medicinal Chemistry,
2019
.

Bauer A.W., Kirby W.M., Sherris J.C., Turck M.
American Journal of Clinical Pathology,
1966
.

Lukin A., Kramer J., Hartmann M., Weizel L., Hernandez-Olmos V., Falahati K., Burghardt I., Kalinchenkova N., Bagnyukova D., Zhurilo N., Rautio J., Forsberg M., Ihalainen J., Auriola S., Leppänen J., et. al.
Bioorganic Chemistry,
2018
.

Guardia A., Baiget J., Cacho M., Pérez A., Ortega-Guerra M., Nxumalo W., Khanye S.D., Rullas J., Ortega F., Jiménez E., Pérez-Herrán E., Fraile-Gabaldón M.T., Esquivias J., Fernández R., Porras-De Francisco E., et. al.
Journal of Medicinal Chemistry,
2018
.

Lukin A., Chudinov M., Vedekhina T., Rogacheva E., Kraeva L., Bakulina O., Krasavin M.
Molecules,
2022
.

He J., Wang C., Zhu Y., Ai D.
Journal of Diabetes,
2016
.

Serral F., Castello F.A., Sosa E.J., Pardo A.M., Palumbo M.C., Modenutti C., Palomino M.M., Lazarowski A., Auzmendi J., Ramos P.I., Nicolás M.F., Turjanski A.G., Martí M.A., Fernández Do Porto D.
Frontiers in Pharmacology,
2021
.

Iyer M.R., Kundu B., Wood C.M.
Expert Opinion on Therapeutic Patents,
2022
.

Brown J.R., North E.J., Hurdle J.G., Morisseau C., Scarborough J.S., Sun D., Korduláková J., Scherman M.S., Jones V., Grzegorzewicz A., Crew R.M., Jackson M., McNeil M.R., Lee R.E.
Bioorganic and Medicinal Chemistry,
2011
.

North E.J., Scherman M.S., Bruhn D.F., Scarborough J.S., Maddox M.M., Jones V., Grzegorzewicz A., Yang L., Hess T., Morisseau C., Jackson M., McNeil M.R., Lee R.E.
Bioorganic and Medicinal Chemistry,
2013
.

Matuschek E., Brown D.F., Kahlmeter G.
Clinical Microbiology and Infection,
2014
.

Biswal B.K., Morisseau C., Garen G., Cherney M.M., Garen C., Niu C., Hammock B.D., James M.N.
Journal of Molecular Biology,
2008
.

Krasavin M., Lukin A., Bagnyukova D., Zhurilo N., Zahanich I., Zozulya S.
Journal of Enzyme Inhibition and Medicinal Chemistry,
2016
.

Bellien J., Joannides R.
Journal of Cardiovascular Pharmacology,
2012