Keywords
amidation
antitumor
apoptosis.
drug design
Hantzsch reaction
structure–activity relationship
thiazole derivatives
Abstract
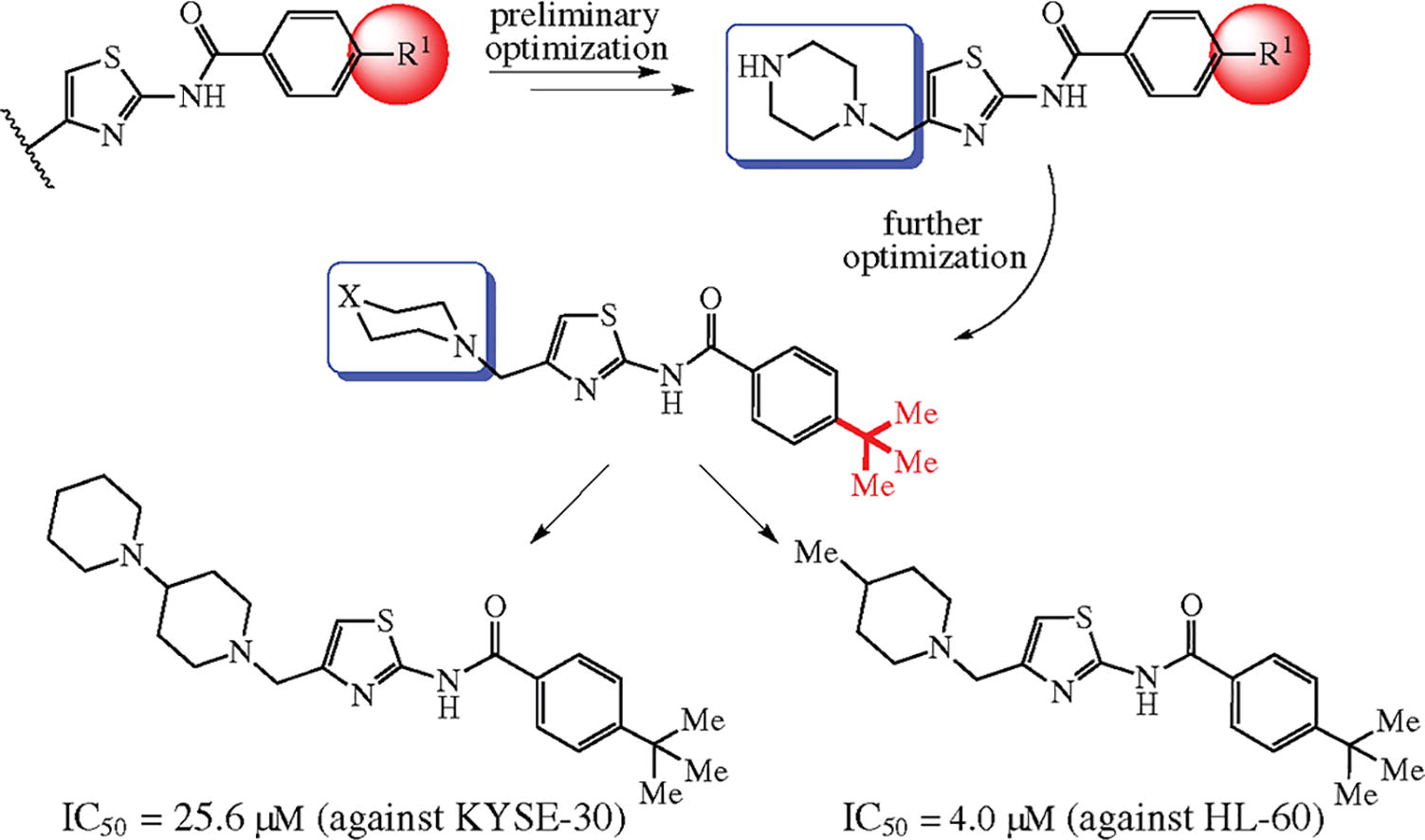
Novel 2-amino-4-(aminomethyl)thiazole derivatives were designed and synthesized by a facile method including the Hantzsch construction of thiazole core followed by amidation and nucleophilic substitution steps. Bioassay results showed that 4-(tert-butyl)-N-[4-(piperazin-1-ylmethyl)thiazol-2-yl]-benzamide and 4-(tert-butyl)-N-{4-[(4-piperidinopiperidin-1-yl)methyl]thiazol-2-yl}benzamide possessed similar activities compared with 5-fluorouracil. The 4-piperidino-piperidin-1-yl-containing derivative also suppressed proliferation of cultured tumor cells by inducing apoptosis.
References
.
![New Antitumor Imidazo[2,1-b]thiazole Guanylhydrazones and Analogues1](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R., Rambaldi M., Varoli L., Calonghi N., Cappadone C., Farruggia G., Zini M., Stefanelli C., Masotti L., Radin N.S., et. al.
Journal of Medicinal Chemistry,
2008
.

Hantzsch A., Weber J.H.
Berichte der deutschen chemischen Gesellschaft,
1887
.

Wu Q., Zhao B., Fan Z., Guo X., Yang D., Zhang N., Yu B., Zhou S., Zhao J., Chen F.
Journal of Agricultural and Food Chemistry,
2019
.
![Synthesis of 3-(Imidazo[2,1-b]thiazol-6-yl)-2H-chromen-2-one Derivatives and Study of Their Antiviral Activity against Parvovirus B19](/storage/images/resized/MjH1ITP7lMYGxeqUZfkt2BnVLgjkk413jwBV97XX_small_thumb.webp)
Conti I., Morigi R., Locatelli A., Rambaldi M., Bua G., Gallinella G., Leoni A.
Molecules,
2019
.

Lee Y.E., Chuang S., Huang L.Y., Lai C., Lin Y., Yang J., Liu C., Yang S., Lin H., Chang C., Lai J., Jian P., Lam K., Chang J., Lau J.Y., et. al.
Journal of Medicinal Chemistry,
2014
.

Christy M.P., Johnson T., McNerlin C.D., Woodard J., Nelson A.T., Lim B., Hamilton T.L., Freiberg K.M., Siegel D.
Organic Letters,
2020
.

Mohareb R.M., Mostafa B.M.
Journal of Heterocyclic Chemistry,
2020
.

Abdel‐Galil E., Girges M.M., Said G.E.
ChemistrySelect,
2020
.

Lalithamba H.S., Uma K., Gowthami T.S., Nagendra G.
Organic Preparations and Procedures International,
2020
.

Mayer I.A., Abramson V., Balko J., Sanders M., Juric D., Solit D., Li Y., Cantley L., Winer E., Arteaga C.
Cancer Research,
2015
.

Chen N., Xie Y., Lu G., Ye F., Li X., Huang Y., Huang M., Chen T., Li C.
Molecular Diversity,
2020
.

Stankova I., Chuchkov K., Shishkov S., Kostova K., Mukova L., Galabov A.S.
Amino Acids,
2008
.
![2-Benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivatives as potent inhibitors of CK1δ/ε](/storage/images/resized/voXLqlsvTwv5p3iMQ8Dhs95nqB4AXOG7Taj7G4ra_small_thumb.webp)
Bischof J., Leban J., Zaja M., Grothey A., Radunsky B., Othersen O., Strobl S., Vitt D., Knippschild U.
Amino Acids,
2012
.

Yan Z., Liu A., Ou Y., Li J., Yi H., Zhang N., Liu M., Huang L., Ren J., Liu W., Hu A.
Bioorganic and Medicinal Chemistry,
2019
.

Zhao J., Quan H., Xu Y., Kong X., Jin L., Lou L.
Cancer Science,
2014
.

Sinha S., Doble M., Manju S.L.
European Journal of Medicinal Chemistry,
2018
.

El-Kerdawy M.M., Ghaly M.A., Darwish S.A., Abdel-Aziz H.A., Elsheakh A.R., Abdelrahman R.S., Hassan G.S.
Bioorganic Chemistry,
2019
.

Maghraby M.T., Abou-Ghadir O.M., Abdel-Moty S.G., Ali A.Y., Salem O.I.
Bioorganic and Medicinal Chemistry,
2020
.

Steinberg M.
Clinical Therapeutics,
2008
.

.

Savage D.G., Antman K.H.
New England Journal of Medicine,
2002
.

Venkatachalam T.K., Sudbeck E.A., Mao C., Uckun F.M.
Bioorganic and Medicinal Chemistry Letters,
2001