Keywords
copper(ii) metallacrowns
DFT calculations
organohalogen compounds
photo- polymerization of dimethacrylate.
salicylhydroxamic acids
X-ray structure
Abstract
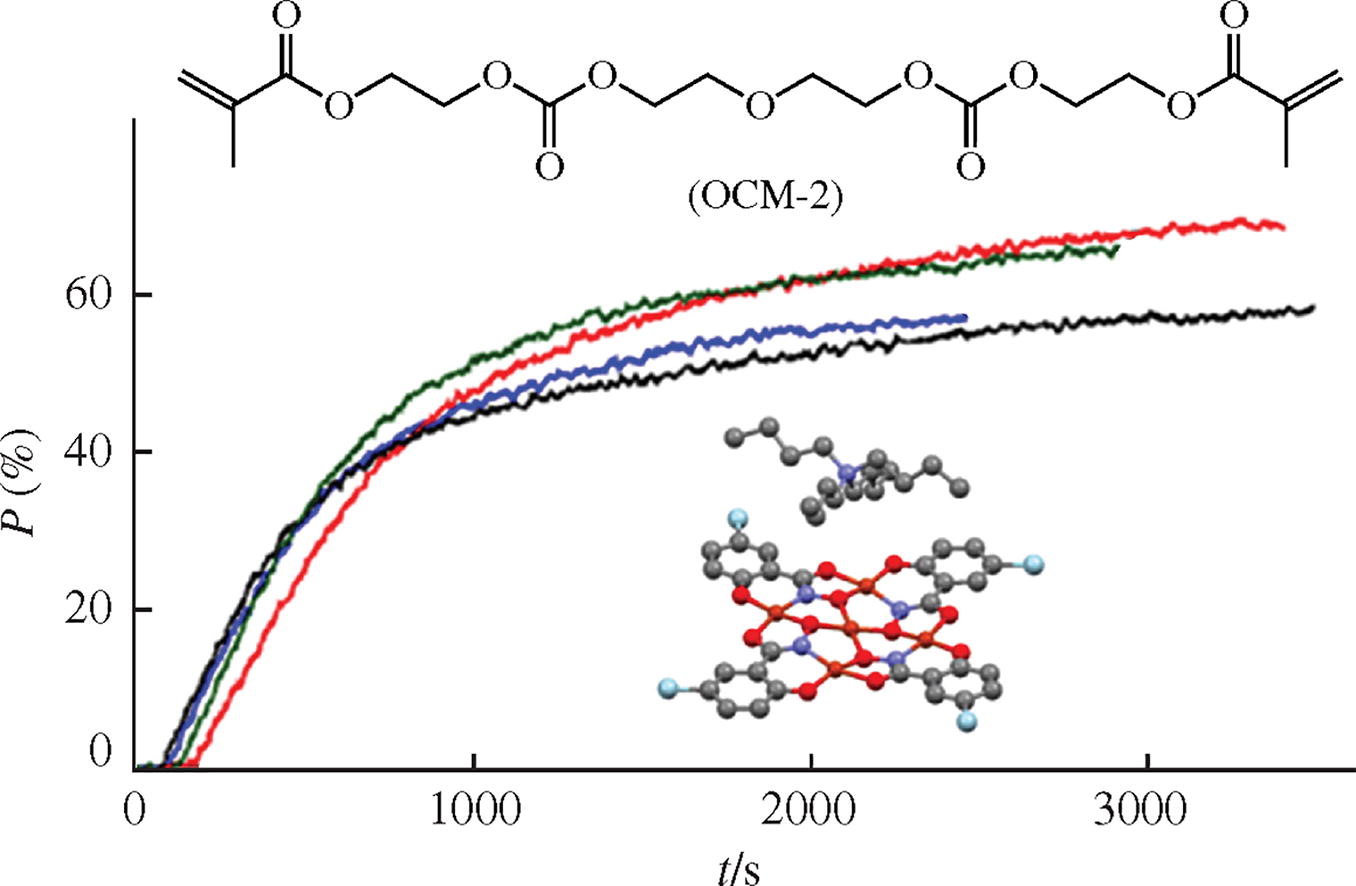
New halogen-substituted salicylhydroximate copper(II) metallacrowns were synthesized from Cu(OAc)2 and the corresponding salicylhydroxamic acids. Their molecular structure was determined by single crystal X-ray diffraction. The compounds obtained represent the first members of the metallacrown family that can be used as components of active initiators for the photopolymerization of oligo-carbonate dimethacrylate by visible light.
References
.

Sheldrick G.M.
Acta crystallographica. Section C, Structural chemistry,
2015
.

Sheldrick G.M.
Acta Crystallographica Section A: Foundations and Advances,
2015
.

Happ P., Plenk C., Rentschler E.
Coordination Chemistry Reviews,
2015
.

Zhigulin G.Y., Zabrodina G.S., Katkova M.A., Ketkov S.Y.
Russian Chemical Bulletin,
2018
.

Lee C., Yang W., Parr R.G.
Physical Review B,
1988
.

Macrae C.F., Sovago I., Cottrell S.J., Galek P.T., McCabe P., Pidcock E., Platings M., Shields G.P., Stevens J.S., Towler M., Wood P.A.
Journal of Applied Crystallography,
2020
.
Zubenko A.D., Egorova B.V., Zamurueva L.S., Kalmykov S.N., Fedorova O.A.
Mendeleev Communications,
2021
.

Stephens P.J., Devlin F.J., Chabalowski C.F., Frisch M.J.
The Journal of Physical Chemistry,
1994
.

Becke A.D.
Journal of Chemical Physics,
1993
.

Krause L., Herbst-Irmer R., Sheldrick G.M., Stalke D.
Journal of Applied Crystallography,
2015
.

Godbout N., Salahub D.R., Andzelm J., Wimmer E.
Canadian Journal of Chemistry,
1992
.

Sosa C., Andzelm J., Elkin B.C., Wimmer E., Dobbs K.D., Dixon D.A.
The Journal of Physical Chemistry,
1992
.

Clark T., Hennemann M., Murray J.S., Politzer P.
Journal of Molecular Modeling,
2006
.

Codd R.
Coordination Chemistry Reviews,
2008
.

Mezei G., Zaleski C.M., Pecoraro V.L.
Chemical Reviews,
2007
.

Tegoni M., Remelli M.
Coordination Chemistry Reviews,
2012
.

Bodwin J.J., Cutland A.D., Malkani R.G., Pecoraro V.L.
Coordination Chemistry Reviews,
2001
.

Ostrowska M., Fritsky I.O., Gumienna-Kontecka E., Pavlishchuk A.V.
Coordination Chemistry Reviews,
2016
.

Katkova M.A., Zabrodina G.S., Baranov E.V., Muravyeva M.S., Kluev E.A., Shavyrin A.S., Zhigulin G.Y., Ketkov S.Y.
Applied Organometallic Chemistry,
2018
.

Katkova M.A.
Russian Journal of Coordination Chemistry/Koordinatsionnaya Khimiya,
2018
.

Gibney B.R., Kessissoglou D.P., Kampf J.W., Pecoraro V.L.
Inorganic Chemistry,
1994
.

Happ P., Rentschler E.
Dalton Transactions,
2014
.

Katkova M.A., Zabrodina G.S., Zhigulin G.Y., Rumyantsev R.V., Ketkov S.Y.
Russian Journal of Coordination Chemistry/Koordinatsionnaya Khimiya,
2019
.

Gupta S.P.
Chemical Reviews,
2015
.

Zhigulin G.Y., Zabrodina G.S., Katkova M.A., Ketkov S.Y.
Russian Journal of Coordination Chemistry/Koordinatsionnaya Khimiya,
2019
.

Plenk C., Krause J., Beck M., Rentschler E.
Chemical Communications,
2015
.
![Isolation and characterization of {MnII[MnIII(salicylhydroximate)]4(acetate)2(DMF)6}.cntdot.2DMF: an inorganic analog of M2+(12-crown-4)](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Lah M.S., Pecoraro V.L.
Journal of the American Chemical Society,
1989
.

Pavlyukh Y., Rentschler E., Elmers H.J., Hübner W., Lefkidis G.
Physical Review B,
2018
.

Zakharina M.Y., Chechet Y.V., Shurygina M.P., Chesnokov S.A.
Polymer Science - Series B,
2018
.

Lah M.S., Pecoraro V.L.
Comments on Inorganic Chemistry,
1990
.

Dendrinou-Samara C., Psomas G., Iordanidis L., Tangoulis V., Kessissoglou D.P.
Chemistry - A European Journal,
2001
.
Panchenko P.A., Fedorov Y.V., Polyakova A.S., Fedorova O.A.
Mendeleev Communications,
2021
.

Veljković D.Ž.
Journal of Molecular Graphics and Modelling,
2018
.
Panchenko P.A., Ignatov P.A., Zakharko M.A., Fedorov Y.V., Fedorova O.A.
Mendeleev Communications,
2020
.
Nguyen L.M., Truong H.H., Khrustalev V.N., Truong S.T., Nguyen D.T., Tran V.T., Mai S.T., Tran V.T., Le A.T.
Mendeleev Communications,
2020