Keywords
aryl hydroxamates
C-H activation
diazo compounds
isoindolones
organofluorine compounds.
rhodium(iii) catalysis
Abstract
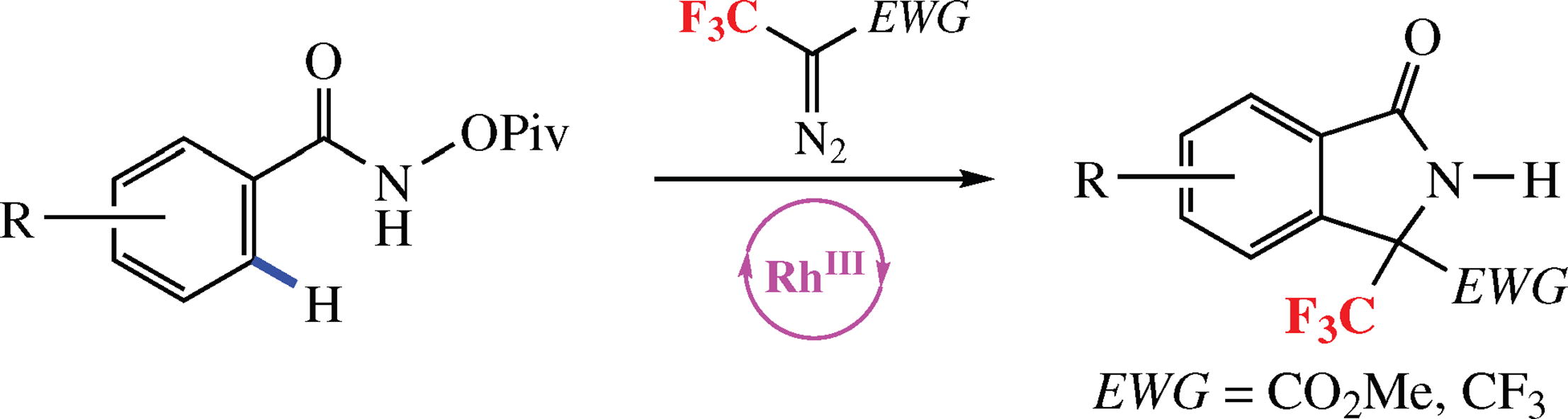
An efficient method for the selective preparation of trifluoro-methyl-substituted isoindolones has been developed via RhIII catalyzed C-H activation / [4 + 1]-annulation of aryl hydroxamates with functionalized acceptor/acceptor diazo compounds as cross-coupling partners.
References
.
Vorob’eva D.V., Titanyuk I.D., Beletskaya I.P., Osipov S.N.
Mendeleev Communications,
2005
.

Nieto S., Sayago F.J., Laborda P., Soler T., Cativiela C., Urriolabeitia E.P.
Tetrahedron,
2011
.

Sambiagio C., Schönbauer D., Blieck R., Dao-Huy T., Pototschnig G., Schaaf P., Wiesinger T., Zia M.F., Wencel-Delord J., Besset T., Maes B.U., Schnürch M.
Chemical Society Reviews,
2018
.

Xia Y., Qiu D., Wang J.
Chemical Reviews,
2017
.
![Synthesis of functionalized CF3-containing heterocycles via [2,3]-sigmatropic rearrangement and sequential catalytic carbocyclization](/storage/images/resized/GDnYOu1UpMMfMMRV6Aqle4H0YLLsraeD9IP9qScG_small_thumb.webp)
Vorobyeva D.V., Mailyan A.K., Peregudov A.S., Karimova N.M., Vasilyeva T.P., Bushmarinov I.S., Bruneau C., Dixneuf P.H., Osipov S.N.
Tetrahedron,
2011
.

Hyster T.K., Ruhl K.E., Rovis T.
Journal of the American Chemical Society,
2013
.

Osipov S.N., Sewald N., Kolomiets A.F., Fokin A.V., Burger K.
Tetrahedron Letters,
1996
.

Iagafarova I.E., Vorobyeva D.V., Loginov D.A., Peregudov A.S., Osipov S.N.
European Journal of Organic Chemistry,
2017
.

Gandeepan P., Müller T., Zell D., Cera G., Warratz S., Ackermann L.
Chemical Reviews,
2018
.

Chu J.C., Rovis T.
Angewandte Chemie - International Edition,
2017
.

Park Y., Kim Y., Chang S.
Chemical Reviews,
2017
.

Recent advances in positional-selective alkenylations: removable guidance for twofold C–H activation
Ma W., Gandeepan P., Li J., Ackermann L.
Organic Chemistry Frontiers,
2017
.

Gensch T., James M.J., Dalton T., Glorius F.
Angewandte Chemie - International Edition,
2018
.

Hummel J.R., Boerth J.A., Ellman J.A.
Chemical Reviews,
2016
.

Vorobyeva D.V., Vinogradov M.M., Nelyubina Y.V., Loginov D.A., Peregudov A.S., Osipov S.N.
Organic and Biomolecular Chemistry,
2018
.

Mailyan A.K., Krylov I.M., Bruneau C., Dixneuf P.H., Osipov S.N.
European Journal of Organic Chemistry,
2013
.

Louillat M., Patureau F.W.
Chemical Society Reviews,
2014
.
![Cyclobutene Ring-Opening of Bicyclo[4.2.0]octa-1,6-dienes: Access to CF3-Substituted 5,6,7,8-Tetrahydro-1,7-naphthyridines](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Mailyan A.K., Peregudov A.S., Dixneuf P.H., Bruneau C., Osipov S.N.
Journal of Organic Chemistry,
2012
.

Chen Z., Wang B., Zhang J., Yu W., Liu Z., Zhang Y.
Organic Chemistry Frontiers,
2015
.

Ros A., Fernández R., Lassaletta J.M.
Chemical Society Reviews,
2014
.
Vinogradov M.M., Vorobyeva D.V., Nelyubina Y.V., Osipov S.N., Loginov D.A.
Mendeleev Communications,
2020
.

Tsyshchuk I.E., Vorobyeva D.V., Peregudov A.S., Osipov S.N.
European Journal of Organic Chemistry,
2014
.

Hu F., Xia Y., Ma C., Zhang Y., Wang J.
Chemical Communications,
2015
.

Dong Z., Ren Z., Thompson S.J., Xu Y., Dong G.
Chemical Reviews,
2017
.

Ye B., Cramer N.
Angewandte Chemie - International Edition,
2014
.

Scorzelli F., Di Mola A., De Piano F., Tedesco C., Palombi L., Filosa R., Waser M., Massa A.
Tetrahedron,
2017
.

Wrobel J., Dietrich A., Woolson S.A., Millen J., McCaleb M., Harrison M.C., Hohman T.C., Sredy J., Sullivan D.
Journal of Medicinal Chemistry,
1992
.

Lawson E.C., Luci D.K., Ghosh S., Kinney W.A., Reynolds C.H., Qi J., Smith C.E., Wang Y., Minor L.K., Haertlein B.J., Parry T.J., Damiano B.P., Maryanoff B.E.
Journal of Medicinal Chemistry,
2009
.

Anzini M., Cappelli A., Vomero S., Giorgi G., Langer T., Bruni G., Romeo M.R., Basile A.S.
Journal of Medicinal Chemistry,
1996
.

Scorzelli F., Di Mola A., Palombi L., Massa A.
Molecules,
2015
.

de la Torre B.G., Albericio F.
Molecules,
2021
.

Macsari I., Besidski Y., Csjernyik G., Nilsson L.I., Sandberg L., Yngve U., Åhlin K., Bueters T., Eriksson A.B., Lund P., Venyike E., Oerther S., Hygge Blakeman K., Luo L., Arvidsson P.I., et. al.
Journal of Medicinal Chemistry,
2012
.

Eisenbeis S.A., Chen R., Kang M., Barrila M., Buzon R.
Organic Process Research and Development,
2014
.

Barrio P., Ibáñez I., Herrera L., Román R., Catalán S., Fustero S.
Chemistry - A European Journal,
2015
.

Linev V.V., Kolomiets A.F., Fokin A.V.
Russian Chemical Bulletin,
1992
.
![Rhodium(iii)-catalyzed formal oxidative [4 + 1] cycloaddition of benzohydroxamic acids and α-diazoesters. A facile synthesis of functionalized benzolactams](/storage/images/resized/leiAYcRDGTSl5B1eCnwpSGqmDEUEfDPPoYisFGhT_small_thumb.webp)
Lam H., Man K., Chan W., Zhou Z., Yu W.
Organic and Biomolecular Chemistry,
2014
.

Manikandan R., Jeganmohan M.
Chemical Communications,
2017
.

Chen Z., Rong M., Nie J., Zhu X., Shi B., Ma J.
Chemical Society Reviews,
2019
.

Song L., Fan Z., Zhang A.
Organic and Biomolecular Chemistry,
2019
.
![Selective non-nucleoside HIV-1 reverse transcriptase inhibitors. New 2,3-dihydrothiazolo[2,3-a]isoindol-5(9bH)-ones and related compounds with anti-HIV-1 activity](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Mertens A., Zilch H., Koenig B., Schaefer W., Poll T., Kampe W., Seidel H., Leser U., Leinert H.
Journal of Medicinal Chemistry,
1993
.

Trivedi S., Dekermendjian K., Julien R., Huang J., Lund P., Krupp J., Kronqvist R., Larsson O., Bostwick R.
Assay and Drug Development Technologies,
2007
.

Bishop A.C., Ubersax J.A., Petsch D.T., Matheos D.P., Gray N.S., Blethrow J., Shimizu E., Tsien J.Z., Schultz P.G., Rose M.D., Wood J.L., Morgan D.O., Shokat K.M.
Nature,
2000
.

Massa A., Rizzo P., Scorzelli F., Monaco G., Zanasi R.
Journal of Pharmaceutical and Biomedical Analysis,
2017
.

Pesquet A., Othman M.
Tetrahedron Letters,
2013
.

Scorzelli F., Di Mola A., Palombi L., Filosa R., Massa A.
Synthetic Communications,
2015
.

Rammah M.M., Othman M., Ciamala K., Strohmann C., Rammah M.B.
Tetrahedron,
2008