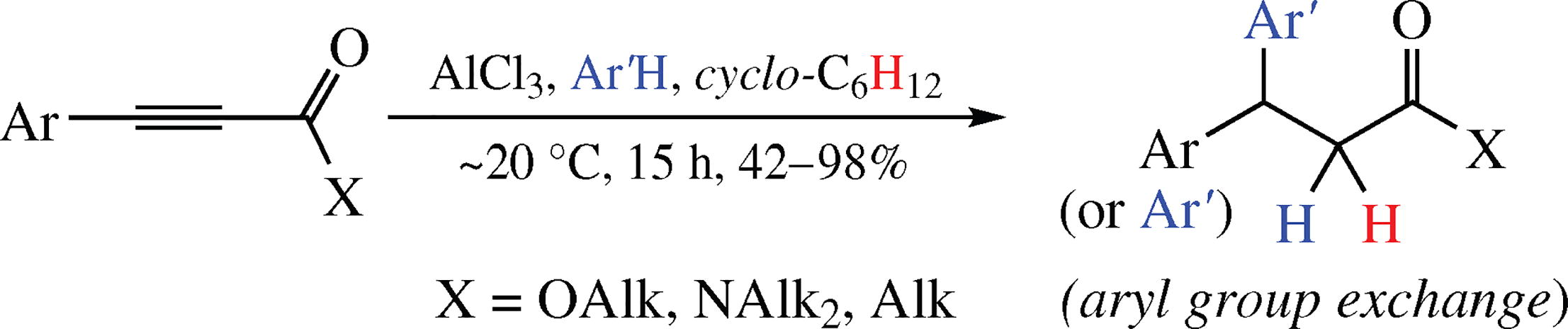Keywords
arylpropynoic acids
carbocations.
hydroarylation
ionic hydrogenation
superelectrophilic activation
ynones
Abstract
Reactions of 3-arylpropynoic acid derivatives and conjugated acetylene ketones with arenes and cyclohexane under the action of AlCl3 at room temperature for 15 h afford the corresponding products of one-pot tandem hydroarylation-ionic hydrogenation of the acetylene bond of the starting compounds in 42-98% yields. This reaction is accompanied by aryl group exchange process under acidic conditions.
References
.

Beletskaya I.P., Nájera C., Yus M.
Russian Chemical Reviews,
2020
.

Zakusilo D.N., Ryabukhin D.S., Boyarskaya I.A., Yuzikhin O.S., Vasilyev A.V.
Tetrahedron,
2015
.

Sandzhieva M.A., Kazakova A.N., Boyarskaya I.A., Ivanov A.Y., Nenajdenko V.G., Vasilyev A.V.
Journal of Organic Chemistry,
2016
.

Michelet B., Bour C., Gandon V.
Chemistry - A European Journal,
2014
.

Borisova M.А., Ryabukhin D.S., Ivanov A.Y., Boyarskaya I.A., Spiridonova D.V., Kompanets M.O., Vasilyev A.V.
Chemistry of Heterocyclic Compounds,
2021
.

Koltunov K., Walspurger S., Sommer J.
European Journal of Organic Chemistry,
2004
.

Koltunov K.Y., Prakash G.K., Rasul G., Olah G.A.
Journal of Organic Chemistry,
2002
.

Gorbunova Y., Zakusilo D.N., Boyarskaya I.A., Vasilyev A.V.
Tetrahedron,
2020
.

Nilov D.I., Vasilyev A.V.
Tetrahedron Letters,
2015
.

Koltunov K.Y., Walspurger S., Sommer J.
Journal of Molecular Catalysis A Chemical,
2006
.

Koltunov K.Y., Prakash G.K., Rasul G., Olah G.A.
Journal of Organic Chemistry,
2002
.

Koltunov K.Y., Prakash G.K., Rasul G., Olah G.A.
Journal of Organic Chemistry,
2007
.

Kochurin M.A., Ismagilova A.R., Zakusilo D.N., Khoroshilova O.V., Boyarskaya I.A., Vasilyev A.V.
New Journal of Chemistry,
2022
.

Rybacka O., Skurski P.
Theoretical Chemistry Accounts,
2018
.

Koltunov K.Y., Walspurger S., Sommer J.
Catalysis Letters,
2004
.

Koltunov K.Y., Repinskaya I.B.
Russian Journal of Organic Chemistry,
2002
.

Koltunov K.Y., Repinskaya I.B., Borodkin G.I.
Russian Journal of Organic Chemistry,
2001
.

Koltunov K.Y., Prakash G.K., Rasul G., Olah G.A.
European Journal of Organic Chemistry,
2006
.

Zhu Z., Ostashevskaya L.A., Koltunov K.Y.
Tetrahedron Letters,
2015
.
Zhu Z., Koltunov K.Y.
Mendeleev Communications,
2020
.

Jacquesy J.
Journal of Fluorine Chemistry,
2006
.

Koltunov K.Y.
Tetrahedron Letters,
2007
.

Jacquesy J., Jacquesy R., Joly G.
Tetrahedron Letters,
1974
.

Lafitte C., Jouannetaud M., Jacquesy J., Fahy J., Duflos A.
Tetrahedron Letters,
1998
.

Koltunov K.Y., Surya Prakash G.K., Rasul G., Olah G.A.
Tetrahedron,
2002