Keywords
acetylenes
ethynylation
imines
pyrroles
pyrrolines.
superbases
vinylation
Abstract
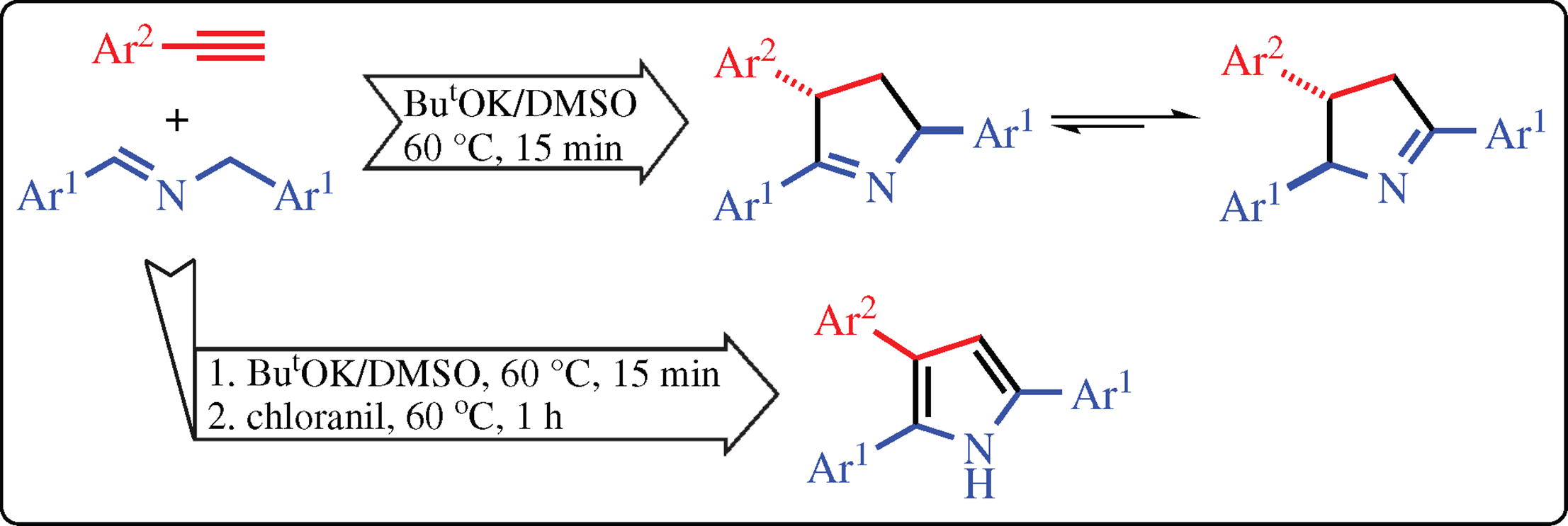
N-Benzyl aldimines react with arylacetylenes in the presence of ButOK/DMSO superbase system to afford 2,3,5-triaryl-1-pyrrolines as two tautomers with 1,2- and 1,5-location of the double bond, both being the trans-diastereomers. This version of the C=N bond ethynylation differs from the previous one with N-benzyl ketimines. The oxidation of the pyrroline tautomeric mixtures without their isolation gives 2,3,5-triaryl-1H-pyrroles.
References
.

Bellina F., Rossi R.
Tetrahedron,
2006
.

Trotuş I., Zimmermann T., Schüth F.
Chemical Reviews,
2013
.
![Base-Catalyzed [3 + 2] Cycloaddition of N-Benzyl Ketimines to Arylacetylenes Followed by Oxidation: A One-Pot Access to Polyarylated 2H-Pyrroles via Intermediate Pyrrolines](/storage/images/resized/iLiQsFqFaSEx6chlGQ5fbAwF6VYU3WWa08hkss0g_small_thumb.webp)
Bidusenko I.A., Schmidt E.Y., Ushakov I.A., Vashchenko A.V., Trofimov B.A.
Organic Letters,
2021
.

Polák P., Tobrman T.
Organic Letters,
2017
.

Yamaguchi M., Fujiwara S., Manabe K.
Organic Letters,
2019
.

Zhuo C., Zhou Y., You S.
Journal of the American Chemical Society,
2014
.

Tang S., Zhang X., Sun J., Niu D., Chruma J.J.
Chemical Reviews,
2018
.

Yang P., You S.
Organic Letters,
2018
.

Bidusenko I.A., Schmidt E.Y., Protsuk N.I., Ushakov I.A., Vashchenko A.V., Afonin A.V., Trofimov B.A.
Organic Letters,
2020
.

Transition-Metal-Free Superbase-Catalyzed C–H Vinylation of Aldimines with Acetylenes to 1-Azadienes
Schmidt E.Y., Bidusenko I.A., Protsuk N.I., Demyanov Y.V., Ushakov I.A., Vashchenko A.V., Trofimov B.A.
Journal of Organic Chemistry,
2020
.

Bidusenko I.A., Schmidt E.Y., Ushakov I.A., Trofimov B.A.
European Journal of Organic Chemistry,
2018
.

Miller S.I., Shkapenko G.
Journal of the American Chemical Society,
1955
.

Bordwell F.G., Drucker G.E., Andersen N.H., Denniston A.D.
Journal of the American Chemical Society,
1986